A vital role for interleukin-21 in the control of a chronic viral infection
- PMID: 19443735
- PMCID: PMC2736049
- DOI: 10.1126/science.1175194
A vital role for interleukin-21 in the control of a chronic viral infection
Abstract
Understanding the factors that regulate the induction, quality, and longevity of antiviral T cell responses is essential for devising rational strategies to prevent or combat infections. In this study, we show that interleukin-21 (IL-21), likely produced by CD4+ T cells, directly influences the generation of polyfunctional CD8+ T cells and that the number of CD4+ T cells that produce IL-21 differs markedly between acute and chronic infections. IL-21 regulates the development of CD8+ T cell exhaustion and the ability to contain chronic lymphocytic choriomeningitis virus infection. Thus, IL-21 serves as a critical helper factor that shapes the functional quality of antiviral CD8+ T cells and is required for viral control.
Figures
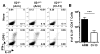
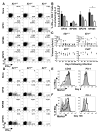


Comment in
-
Immunology. A chronic need for IL-21.Science. 2009 Jun 19;324(5934):1525-6. doi: 10.1126/science.1176487. Science. 2009. PMID: 19541985 No abstract available.
Similar articles
-
IL-21 is required to control chronic viral infection.Science. 2009 Jun 19;324(5934):1569-72. doi: 10.1126/science.1174182. Epub 2009 May 7. Science. 2009. PMID: 19423777 Free PMC article.
-
IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection.J Immunol. 2010 Oct 15;185(8):4835-45. doi: 10.4049/jimmunol.1001032. Epub 2010 Sep 15. J Immunol. 2010. PMID: 20844201 Free PMC article.
-
Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4⁺ T cell responses and viral control during chronic infection.Immunity. 2013 Sep 19;39(3):548-59. doi: 10.1016/j.immuni.2013.08.010. Epub 2013 Aug 29. Immunity. 2013. PMID: 23993651 Free PMC article.
-
T cell responses to viral infections: lessons from lymphocytic choriomeningitis virus.Immunol Res. 2002;26(1-3):309-21. doi: 10.1385/IR:26:1-3:309. Immunol Res. 2002. PMID: 12403369 Review.
-
Induction and maintenance of CD8+ T cells specific for persistent viruses.Adv Exp Med Biol. 2007;590:121-37. doi: 10.1007/978-0-387-34814-8_9. Adv Exp Med Biol. 2007. PMID: 17191382 Review. No abstract available.
Cited by
-
IL-21 promotes the pathologic immune response to pneumovirus infection.J Immunol. 2012 Feb 15;188(4):1924-32. doi: 10.4049/jimmunol.1100767. Epub 2012 Jan 11. J Immunol. 2012. PMID: 22238461 Free PMC article.
-
Revealing the role of CD4(+) T cells in viral immunity.J Exp Med. 2012 Jul 30;209(8):1391-5. doi: 10.1084/jem.20121517. J Exp Med. 2012. PMID: 22851641 Free PMC article. Review.
-
Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection.Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21182-7. doi: 10.1073/pnas.1118450109. Epub 2011 Dec 12. Proc Natl Acad Sci U S A. 2011. PMID: 22160724 Free PMC article.
-
N-linked glycans: an underappreciated key determinant of T cell development, activation, and function.Immunometabolism (Cobham). 2023 Nov 21;5(4):e00035. doi: 10.1097/IN9.0000000000000035. eCollection 2023 Oct. Immunometabolism (Cobham). 2023. PMID: 38027254 Free PMC article. Review.
-
IL-21 restricts virus-driven Treg cell expansion in chronic LCMV infection.PLoS Pathog. 2013;9(5):e1003362. doi: 10.1371/journal.ppat.1003362. Epub 2013 May 16. PLoS Pathog. 2013. PMID: 23696736 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

