Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush
- PMID: 19439606
- PMCID: PMC2883456
- DOI: 10.1523/JNEUROSCI.5885-08.2009
Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush
Abstract
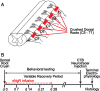
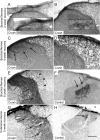
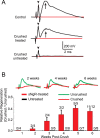
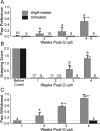
A major impediment for regeneration of axons within the CNS is the presence of multiple inhibitory factors associated with myelin. Three of these factors bind to the Nogo receptor, NgR, which is expressed on axons. Administration of exogenous blockers of NgR or NgR ligands promotes the regeneration of descending axonal projections after spinal cord hemisection. A more detailed analysis of CNS regeneration can be made by examining the growth of specific classes of sensory axons into the spinal cord after dorsal root crush injury. In this study, we assessed whether administration of a soluble peptide fragment of the NgR (sNgR) that binds to and blocks all three NgR ligands can promote regeneration after brachial dorsal root crush in adult rats. Intraventricular infusion of sNgR for 1 month results in extensive regrowth of myelinated sensory axons into the white and gray matter of the dorsal spinal cord, but unmyelinated sensory afferents do not regenerate. In concert with the anatomical growth of sensory axons into the cord, there is a gradual restoration of synaptic function in the denervated region, as revealed by extracellular microelectrode recordings from the spinal gray matter in response to stimulation of peripheral nerves. These positive synaptic responses are correlated with substantial improvements in use of the forelimb, as assessed by paw preference, paw withdrawal to tactile stimuli and the ability to grasp. These results suggest that sNgR may be a potential therapy for restoring sensory function after injuries to sensory roots.
Figures


Similar articles
-
Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat.Neurorehabil Neural Repair. 2008 May-Jun;22(3):262-78. doi: 10.1177/1545968307308550. Epub 2007 Nov 30. Neurorehabil Neural Repair. 2008. PMID: 18056009 Free PMC article.
-
Topographically specific regeneration of sensory axons in the spinal cord.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11585-90. doi: 10.1073/pnas.1003287107. Epub 2010 Jun 4. Proc Natl Acad Sci U S A. 2010. PMID: 20534446 Free PMC article.
-
Soluble Nogo receptor down-regulates expression of neuronal Nogo-A to enhance axonal regeneration.J Biol Chem. 2010 Jan 22;285(4):2783-95. doi: 10.1074/jbc.M109.046425. Epub 2009 Nov 9. J Biol Chem. 2010. PMID: 19901030 Free PMC article.
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
-
Mechanisms of CNS myelin inhibition: evidence for distinct and neuronal cell type specific receptor systems.Restor Neurol Neurosci. 2008;26(2-3):97-115. Restor Neurol Neurosci. 2008. PMID: 18820405 Free PMC article. Review.
Cited by
-
Soluble NgR fusion protein modulates the proliferation of neural progenitor cells via the Notch pathway.Neurochem Res. 2011 Dec;36(12):2363-72. doi: 10.1007/s11064-011-0562-7. Epub 2011 Aug 7. Neurochem Res. 2011. PMID: 21822922 Free PMC article.
-
Artemin promotes functional long-distance axonal regeneration to the brainstem after dorsal root crush.Proc Natl Acad Sci U S A. 2015 May 12;112(19):6170-5. doi: 10.1073/pnas.1502057112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918373 Free PMC article.
-
Sensory axon regeneration: rebuilding functional connections in the spinal cord.Trends Neurosci. 2012 Mar;35(3):156-63. doi: 10.1016/j.tins.2011.10.006. Epub 2011 Nov 30. Trends Neurosci. 2012. PMID: 22137336 Free PMC article. Review.
-
The axon-glia unit in white matter stroke: mechanisms of damage and recovery.Brain Res. 2015 Oct 14;1623:123-34. doi: 10.1016/j.brainres.2015.02.019. Epub 2015 Feb 20. Brain Res. 2015. PMID: 25704204 Free PMC article. Review.
-
Combination treatment of experimental stroke with Niaspan and Simvastatin, reduces axonal damage and improves functional outcome.J Neurol Sci. 2010 Jul 15;294(1-2):107-11. doi: 10.1016/j.jns.2010.03.020. Epub 2010 May 7. J Neurol Sci. 2010. PMID: 20451219 Free PMC article.
References
-
- Anderson KD, Gunawan A, Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp Neurol. 2007;206:318–331. - PubMed
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. - PubMed
-
- Ballermann M, McKenna J, Whishaw IQ. A grasp-related deficit in tactile discrimination following dorsal column lesion in the rat. Brain Res Bull. 2001;54:237–242. - PubMed
-
- Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

