A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells
- PMID: 19439586
- PMCID: PMC2752970
- DOI: 10.1523/JNEUROSCI.0132-09.2009
A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells
Abstract
On bipolar cells are connected to photoreceptors via a sign-inverting synapse. At this synapse, glutamate binds to a metabotropic receptor which couples to the closure of a cation-selective transduction channel. The molecular identity of both the receptor and the G protein are known, but the identity of the transduction channel has remained elusive. Here, we show that the transduction channel in mouse rod bipolar cells, a subtype of On bipolar cell, is likely to be a member of the TRP family of channels. To evoke a transduction current, the metabotropic receptor antagonist LY341495 was applied to the dendrites of cells that were bathed in a solution containing the mGluR6 agonists L-AP4 or glutamate. The transduction current was suppressed by ruthenium red and the TRPV1 antagonists capsazepine and SB-366791. Furthermore, focal application of the TRPV1 agonists capsaicin and anandamide evoked a transduction-like current. The capsaicin-evoked and endogenous transduction current displayed prominent outward rectification, a property of the TRPV1 channel. To test the possibility that the transduction channel is TRPV1, we measured rod bipolar cell function in the TRPV1(-/-) mouse. The ERG b-wave, a measure of On bipolar cell function, as well as the transduction current and the response to TRPV1 agonists were normal, arguing against a role for TRPV1. However, ERG measurements from mice lacking TRPM1 receptors, another TRP channel implicated in retinal function, revealed the absence of a b-wave. Our results suggest that a TRP-like channel, possibly TRPM1, is essential for synaptic function in On bipolar cells.
Figures

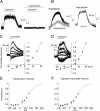
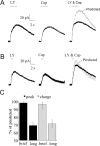
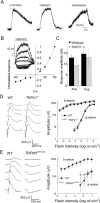
Comment in
-
Role of melastatin-related transient receptor potential channel TRPM1 in the retina: Clues from horses and mice.J Neurosci. 2009 Sep 23;29(38):11720-2. doi: 10.1523/JNEUROSCI.3275-09.2009. J Neurosci. 2009. PMID: 19776258 Free PMC article. No abstract available.
Similar articles
-
G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gβγ dimer.Proc Natl Acad Sci U S A. 2012 May 29;109(22):8752-7. doi: 10.1073/pnas.1117433109. Epub 2012 May 14. Proc Natl Acad Sci U S A. 2012. PMID: 22586107 Free PMC article.
-
GPR179 is required for high sensitivity of the mGluR6 signaling cascade in depolarizing bipolar cells.J Neurosci. 2014 Apr 30;34(18):6334-43. doi: 10.1523/JNEUROSCI.4044-13.2014. J Neurosci. 2014. PMID: 24790204 Free PMC article.
-
TRPV1 channels facilitate glutamate transmission in the striatum.Mol Cell Neurosci. 2009 Jan;40(1):89-97. doi: 10.1016/j.mcn.2008.09.001. Epub 2008 Sep 30. Mol Cell Neurosci. 2009. PMID: 18930149
-
TRPM1.Handb Exp Pharmacol. 2014;222:387-402. doi: 10.1007/978-3-642-54215-2_15. Handb Exp Pharmacol. 2014. PMID: 24756714 Review.
-
Transient Receptor Potential (TRP) Channels in Pain, Neuropsychiatric Disorders, and Epilepsy.Int J Mol Sci. 2023 Mar 1;24(5):4714. doi: 10.3390/ijms24054714. Int J Mol Sci. 2023. PMID: 36902145 Free PMC article. Review.
Cited by
-
TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells.Proc Natl Acad Sci U S A. 2009 Nov 10;106(45):19174-8. doi: 10.1073/pnas.0908711106. Epub 2009 Oct 27. Proc Natl Acad Sci U S A. 2009. PMID: 19861548 Free PMC article.
-
Expression and distribution of trophoblast glycoprotein in the mouse retina.J Comp Neurol. 2020 Jul;528(10):1660-1671. doi: 10.1002/cne.24850. Epub 2020 Jan 6. J Comp Neurol. 2020. PMID: 31891182 Free PMC article.
-
R9AP stabilizes RGS11-G beta5 and accelerates the early light response of ON-bipolar cells.Vis Neurosci. 2010 Mar;27(1-2):9-17. doi: 10.1017/S0952523809990319. Epub 2010 Jan 26. Vis Neurosci. 2010. PMID: 20100392 Free PMC article.
-
TRPM channels in health and disease.Nat Rev Nephrol. 2024 Mar;20(3):175-187. doi: 10.1038/s41581-023-00777-y. Epub 2023 Oct 18. Nat Rev Nephrol. 2024. PMID: 37853091 Review.
-
A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites.J Neurosci. 2011 Jul 6;31(27):10060-6. doi: 10.1523/JNEUROSCI.1014-11.2011. J Neurosci. 2011. PMID: 21734298 Free PMC article.
References
-
- Bellone RR, Brooks SA, Sandmeyer L, Murphy BA, Forsyth G, Archer S, Bailey E, Grahn B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the appaloosa horse (Equus caballus) Genetics. 2008;179:1861–1870. - PMC - PubMed
-
- Berntson A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004;21:913–924. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
-
- Clapham DE. SnapShot: mammalian TRP channels. Cell. 2007;129:220, e220–e222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
