The fowlpox virus BCL-2 homologue, FPV039, interacts with activated Bax and a discrete subset of BH3-only proteins to inhibit apoptosis
- PMID: 19439472
- PMCID: PMC2704773
- DOI: 10.1128/JVI.00437-09
The fowlpox virus BCL-2 homologue, FPV039, interacts with activated Bax and a discrete subset of BH3-only proteins to inhibit apoptosis
Abstract
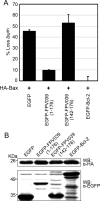
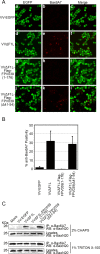
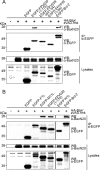
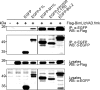
Apoptosis is a potent immune barrier against viral infection, and many viruses, including poxviruses, encode proteins to overcome this defense. Interestingly, the avipoxviruses, which include fowlpox and canarypox virus, are the only poxviruses known to encode proteins with obvious Bcl-2 sequence homology. We previously characterized the fowlpox virus protein FPV039 as a Bcl-2-like antiapoptotic protein that inhibits apoptosis by interacting with and inactivating the proapoptotic cellular protein Bak. However, both Bak and Bax can independently trigger cell death. Thus, to effectively inhibit apoptosis, a number of viruses also inhibit Bax. Here we show that FPV039 inhibited apoptosis induced by Bax overexpression and prevented both the conformational activation of Bax and the subsequent formation of Bax oligomers at the mitochondria, two critical steps in the induction of apoptosis. Additionally, FPV039 interacted with activated Bax in the context of Bax overexpression and virus infection. Importantly, the ability of FPV039 to interact with active Bax and inhibit Bax activity was dependent on the structurally conserved BH3 domain of FPV039, even though this domain possesses little sequence homology to other BH3 domains. FPV039 also inhibited apoptosis induced by the BH3-only proteins, upstream activators of Bak and Bax, despite interacting detectably with only two: BimL and Bik. Collectively, our data suggest that FPV039 inhibits apoptosis by sequestering and inactivating multiple proapoptotic Bcl-2 proteins, including certain BH3-only proteins and both of the critical "gatekeepers" of apoptosis, Bak and Bax.
Figures






Similar articles
-
Fowlpox virus encodes a Bcl-2 homologue that protects cells from apoptotic death through interaction with the proapoptotic protein Bak.J Virol. 2007 Oct;81(20):11032-45. doi: 10.1128/JVI.00734-07. Epub 2007 Aug 8. J Virol. 2007. PMID: 17686864 Free PMC article.
-
Structural basis of apoptosis inhibition by the fowlpox virus protein FPV039.J Biol Chem. 2017 Jun 2;292(22):9010-9021. doi: 10.1074/jbc.M116.768879. Epub 2017 Apr 14. J Biol Chem. 2017. PMID: 28411240 Free PMC article.
-
Deerpox virus encodes an inhibitor of apoptosis that regulates Bak and Bax.J Virol. 2011 Mar;85(5):1922-34. doi: 10.1128/JVI.01959-10. Epub 2010 Dec 15. J Virol. 2011. PMID: 21159883 Free PMC article.
-
BCL-2 proteins and apoptosis: Recent insights and unknowns.Biochem Biophys Res Commun. 2018 May 27;500(1):26-34. doi: 10.1016/j.bbrc.2017.06.190. Epub 2017 Jul 1. Biochem Biophys Res Commun. 2018. PMID: 28676391 Review.
-
BH3-only proteins: a 20-year stock-take.FEBS J. 2015 Mar;282(6):1006-16. doi: 10.1111/febs.13190. Epub 2015 Jan 26. FEBS J. 2015. PMID: 25565426 Review.
Cited by
-
Molecular genetic analysis of orf virus: a poxvirus that has adapted to skin.Viruses. 2015 Mar 23;7(3):1505-39. doi: 10.3390/v7031505. Viruses. 2015. PMID: 25807056 Free PMC article. Review.
-
Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis.Viruses. 2017 Oct 20;9(10):305. doi: 10.3390/v9100305. Viruses. 2017. PMID: 29053589 Free PMC article.
-
Sheeppox virus SPPV14 encodes a Bcl-2-like cell death inhibitor that counters a distinct set of mammalian proapoptotic proteins.J Virol. 2012 Nov;86(21):11501-11. doi: 10.1128/JVI.01115-12. Epub 2012 Aug 15. J Virol. 2012. PMID: 22896610 Free PMC article.
-
Poxviruses and the evolution of host range and virulence.Infect Genet Evol. 2014 Jan;21:15-40. doi: 10.1016/j.meegid.2013.10.014. Epub 2013 Oct 24. Infect Genet Evol. 2014. PMID: 24161410 Free PMC article. Review.
-
Mechanism of the promotion of steatotic HepG2 cell apoptosis by cholesterol.Int J Clin Exp Pathol. 2014 Sep 15;7(10):6807-13. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25400762 Free PMC article.
References
-
- Antonsson, B., S. Montessuit, B. Sanchez, and J. C. Martinou. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 27611615-11623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

