Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm
- PMID: 19439467
- PMCID: PMC2704778
- DOI: 10.1128/JVI.00110-09
Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm
Abstract
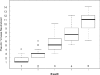
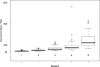
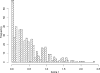
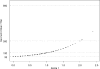
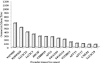
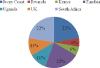
The development of a rapid and efficient system to identify human immunodeficiency virus type 1 (HIV-1)-infected individuals with broad and potent HIV-1-specific neutralizing antibody responses is an important step toward the discovery of critical neutralization targets for rational AIDS vaccine design. In this study, samples from HIV-1-infected volunteers from diverse epidemiological regions were screened for neutralization responses using pseudovirus panels composed of clades A, B, C, and D and circulating recombinant forms (CRFs). Initially, 463 serum and plasma samples from Australia, Rwanda, Uganda, the United Kingdom, and Zambia were screened to explore neutralization patterns and selection ranking algorithms. Samples were identified that neutralized representative isolates from at least four clade/CRF groups with titers above prespecified thresholds and ranked based on a weighted average of their log-transformed neutralization titers. Linear regression methods selected a five-pseudovirus subset, representing clades A, B, and C and one CRF01_AE, that could identify top-ranking samples with 50% inhibitory concentration (IC(50)) neutralization titers of >or=100 to multiple isolates within at least four clade groups. This reduced panel was then used to screen 1,234 new samples from the Ivory Coast, Kenya, South Africa, Thailand, and the United States, and 1% were identified as elite neutralizers. Elite activity is defined as the ability to neutralize, on average, more than one pseudovirus at an IC(50) titer of 300 within a clade group and across at least four clade groups. These elite neutralizers provide promising starting material for the isolation of broadly neutralizing monoclonal antibodies to assist in HIV-1 vaccine design.
Figures

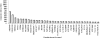

Similar articles
-
Regional clustering of shared neutralization determinants on primary isolates of clade C human immunodeficiency virus type 1 from South Africa.J Virol. 2002 Mar;76(5):2233-44. doi: 10.1128/jvi.76.5.2233-2244.2002. J Virol. 2002. PMID: 11836401 Free PMC article.
-
Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies.J Virol. 2014 Nov;88(21):12623-43. doi: 10.1128/JVI.01705-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142591 Free PMC article.
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
An HIV-1 Broadly Neutralizing Antibody from a Clade C-Infected Pediatric Elite Neutralizer Potently Neutralizes the Contemporaneous and Autologous Evolving Viruses.J Virol. 2019 Feb 5;93(4):e01495-18. doi: 10.1128/JVI.01495-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30429339 Free PMC article.
-
Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers.Retrovirology. 2018 Sep 5;15(1):61. doi: 10.1186/s12977-018-0443-0. Retrovirology. 2018. PMID: 30185183 Free PMC article. Review.
Cited by
-
Serum neutralizing activities from a Beijing homosexual male cohort infected with different subtypes of HIV-1 in China.PLoS One. 2012;7(10):e47548. doi: 10.1371/journal.pone.0047548. Epub 2012 Oct 18. PLoS One. 2012. PMID: 23094060 Free PMC article.
-
Determinants of HIV-1 broadly neutralizing antibody induction.Nat Med. 2016 Nov;22(11):1260-1267. doi: 10.1038/nm.4187. Epub 2016 Sep 26. Nat Med. 2016. PMID: 27668936
-
Affinity for the Interface Underpins Potency of Antibodies Operating In Membrane Environments.Cell Rep. 2020 Aug 18;32(7):108037. doi: 10.1016/j.celrep.2020.108037. Cell Rep. 2020. PMID: 32814041 Free PMC article.
-
Sequential evolution and escape from neutralization of simian immunodeficiency virus SIVsmE660 clones in rhesus macaques.J Virol. 2012 Aug;86(16):8835-47. doi: 10.1128/JVI.00923-12. Epub 2012 Jun 13. J Virol. 2012. PMID: 22696650 Free PMC article.
-
Unique C2V3 sequence in HIV-1 envelope obtained from broadly neutralizing plasma of a slow progressing patient conferred enhanced virus neutralization.PLoS One. 2012;7(10):e46713. doi: 10.1371/journal.pone.0046713. Epub 2012 Oct 3. PLoS One. 2012. PMID: 23056416 Free PMC article.
References
-
- Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 8211651-11668. - PMC - PubMed
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. - PMC - PubMed
-
- Burton, D. R., and C. F. Barbas III. 1994. Human antibodies from combinatorial libraries. Adv. Immunol. 57191-280. - PubMed
-
- Burton, D. R., C. F. Barbas III, M. A. Persson, S. Koenig, R. M. Chanock, and R. A. Lerner. 1991. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. USA 8810134-10137. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

