LISP1 is important for the egress of Plasmodium berghei parasites from liver cells
- PMID: 19438514
- PMCID: PMC2774474
- DOI: 10.1111/j.1462-5822.2009.01333.x
LISP1 is important for the egress of Plasmodium berghei parasites from liver cells
Abstract
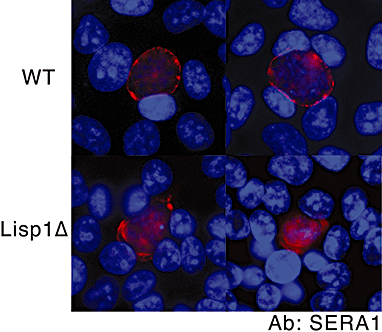
Most Apicomplexa are obligatory intracellular parasites that multiply inside a so-called parasitophorous vacuole (PV) formed upon parasite entry into the host cell. Plasmodium, the agent of malaria and the Apicomplexa most deadly to humans, multiplies in both hepatocytes and erythrocytes in the mammalian host. Although much has been learned on how Apicomplexa parasites invade host cells inside a PV, little is known of how they rupture the PV membrane and egress host cells. Here, we characterize a Plasmodium protein, called LISP1 (liver-specific protein 1), which is specifically involved in parasite egress from hepatocytes. LISP1 is expressed late during parasite development inside hepatocytes and locates at the PV membrane. Intracellular parasites deficient in LISP1 develop into hepatic merozoites, which display normal infectivity to erythrocytes. However, LISP1-deficient liver-stage parasites do not rupture the membrane of the PV and remain trapped inside hepatocytes. LISP1 is the first Plasmodium protein shown by gene targeting to be involved in the lysis of the PV membrane.
Figures


Similar articles
-
Plasmodium berghei EXP-1 interacts with host Apolipoprotein H during Plasmodium liver-stage development.Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):E1138-E1147. doi: 10.1073/pnas.1606419114. Epub 2017 Jan 30. Proc Natl Acad Sci U S A. 2017. PMID: 28137845 Free PMC article.
-
A key role for Plasmodium subtilisin-like SUB1 protease in egress of malaria parasites from host hepatocytes.J Biol Chem. 2013 Nov 15;288(46):33336-46. doi: 10.1074/jbc.M113.513234. Epub 2013 Oct 2. J Biol Chem. 2013. PMID: 24089525 Free PMC article.
-
LC3B labeling of the parasitophorous vacuole membrane of Plasmodium berghei liver stage parasites depends on the V-ATPase and ATG16L1.Mol Microbiol. 2024 Jun;121(6):1095-1111. doi: 10.1111/mmi.15259. Epub 2024 Apr 4. Mol Microbiol. 2024. PMID: 38574236
-
Moving on: How malaria parasites exit the liver.Mol Microbiol. 2024 Mar;121(3):328-340. doi: 10.1111/mmi.15141. Epub 2023 Aug 21. Mol Microbiol. 2024. PMID: 37602900 Review.
-
Molecular make-up of the Plasmodium parasitophorous vacuolar membrane.Int J Med Microbiol. 2012 Oct;302(4-5):179-86. doi: 10.1016/j.ijmm.2012.07.011. Epub 2012 Aug 13. Int J Med Microbiol. 2012. PMID: 22898489 Review.
Cited by
-
Plasmodium berghei Δp52&p36 parasites develop independent of a parasitophorous vacuole membrane in Huh-7 liver cells.PLoS One. 2012;7(12):e50772. doi: 10.1371/journal.pone.0050772. Epub 2012 Dec 5. PLoS One. 2012. PMID: 23227206 Free PMC article.
-
Overlapping and distinct roles of CDPK family members in the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium berghei.PLoS Pathog. 2020 Aug 31;16(8):e1008131. doi: 10.1371/journal.ppat.1008131. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866196 Free PMC article.
-
Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites.Mol Microbiol. 2011 Sep;81(6):1511-25. doi: 10.1111/j.1365-2958.2011.07787.x. Epub 2011 Aug 17. Mol Microbiol. 2011. PMID: 21848587 Free PMC article.
-
Laser capture microdissection enables transcriptomic analysis of dividing and quiescent liver stages of Plasmodium relapsing species.Cell Microbiol. 2017 Aug;19(8):e12735. doi: 10.1111/cmi.12735. Epub 2017 Mar 13. Cell Microbiol. 2017. PMID: 28256794 Free PMC article.
-
Role of Plasmodium berghei cGMP-dependent protein kinase in late liver stage development.J Biol Chem. 2010 Jan 29;285(5):3282-8. doi: 10.1074/jbc.M109.070367. Epub 2009 Nov 24. J Biol Chem. 2010. PMID: 19940133 Free PMC article.
References
-
- Aikawa M. Parasitological review. Plasmodium: the fine structure of malarial parasites. Exp Parasitol. 1971;30:284–320. - PubMed
-
- Arisue N, Hirai M, Arai M, Matsuoka H, Horii T. Phylogeny and evolution of the SERA multigene family in the genus Plasmodium. J Mol Evol. 2007;65:82–91. - PubMed
-
- Bano N, Romano JD, Jayabalasingham B, Coppens I. Cellular interactions of Plasmodium liver stage with its host mammalian cell. Int J Parasitol. 2007;37:1329–1341. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

