Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy
- PMID: 19436756
- PMCID: PMC2677735
- DOI: 10.1371/journal.pone.0005515
Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy
Abstract
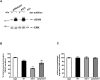
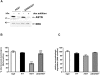
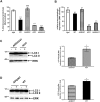
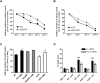
Background: The mechanisms through which aberrant alpha-synuclein (ASYN) leads to neuronal death in Parkinson's disease (PD) are uncertain. In isolated liver lysosomes, mutant ASYNs impair Chaperone Mediated Autophagy (CMA), a targeted lysosomal degradation pathway; however, whether this occurs in a cellular context, and whether it mediates ASYN toxicity, is unknown. We have investigated presently the effects of WT or mutant ASYN on the lysosomal pathways of CMA and macroautophagy in neuronal cells and assessed their impact on ASYN-mediated toxicity.
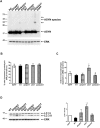
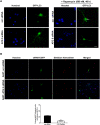
Methods and findings: Novel inducible SH-SY5Y and PC12 cell lines expressing human WT and A53T ASYN, as well as two mutant forms that lack the CMA-targeting motif were generated. Such forms were also expressed in primary cortical neurons, using adenoviral transduction. In each case, effects on long-lived protein degradation, LC3 II levels (as a macroautophagy index), and cell death and survival were assessed. In both PC12 and SH-SY5Y cycling cells, induction of A53T ASYN evoked a significant decrease in lysosomal degradation, largely due to CMA impairment. In neuronally differentiated SH-SH5Y cells, both WT and A53T ASYN induction resulted in gradual toxicity, which was partly dependent on CMA impairment and compensatory macroautophagy induction. In primary neurons both WT and A53T ASYN were toxic, but only in the case of A53T ASYN did CMA dysfunction and compensatory macroautophagy induction occur and participate in death.
Conclusions: Expression of mutant A53T, and, in some cases, WT ASYN in neuronal cells leads to CMA dysfunction, and this in turn leads to compensatory induction of macroautophagy. Inhibition of these lysosomal effects mitigates ASYN toxicity. Therefore, CMA dysfunction mediates aberrant ASYN toxicity, and may be a target for therapeutic intervention in PD and related disorders. Furthermore, macroautophagy induction in the context of ASYN over-expression, in contrast to other settings, appears to be a detrimental response, leading to neuronal death.
Conflict of interest statement
Figures
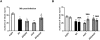
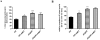
Similar articles
-
Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells.J Biol Chem. 2008 Aug 29;283(35):23542-56. doi: 10.1074/jbc.M801992200. Epub 2008 Jun 19. J Biol Chem. 2008. PMID: 18566453 Free PMC article.
-
Age-dependent accumulation of oligomeric SNCA/α-synuclein from impaired degradation in mutant LRRK2 knockin mouse model of Parkinson disease: role for therapeutic activation of chaperone-mediated autophagy (CMA).Autophagy. 2020 Feb;16(2):347-370. doi: 10.1080/15548627.2019.1603545. Epub 2019 Apr 14. Autophagy. 2020. PMID: 30983487 Free PMC article.
-
alpha-synuclein degradation by autophagic pathways: a potential key to Parkinson's disease pathogenesis.Autophagy. 2008 Oct;4(7):917-9. doi: 10.4161/auto.6685. Epub 2008 Oct 26. Autophagy. 2008. PMID: 18708765
-
Autophagy and Alpha-Synuclein: Relevance to Parkinson's Disease and Related Synucleopathies.Mov Disord. 2016 Feb;31(2):178-92. doi: 10.1002/mds.26477. Epub 2016 Jan 27. Mov Disord. 2016. PMID: 26813776 Review.
-
A new perspective in Parkinson's disease, chaperone-mediated autophagy.Parkinsonism Relat Disord. 2011 May;17(4):231-5. doi: 10.1016/j.parkreldis.2010.12.008. Epub 2011 Jan 7. Parkinsonism Relat Disord. 2011. PMID: 21215675 Review.
Cited by
-
Impairment of chaperone-mediated autophagy induces dopaminergic neurodegeneration in rats.Autophagy. 2016 Nov;12(11):2230-2247. doi: 10.1080/15548627.2016.1214777. Epub 2016 Aug 19. Autophagy. 2016. PMID: 27541985 Free PMC article.
-
Neuronal autophagy, α-synuclein clearance, and LRRK2 regulation: a lost equilibrium in parkinsonian brain.J Neurosci. 2012 Oct 24;32(43):14851-3. doi: 10.1523/JNEUROSCI.3588-12.2012. J Neurosci. 2012. PMID: 23100407 Free PMC article. No abstract available.
-
Macroautophagy in sporadic and the genetic form of Parkinson's disease with the A53T α-synuclein mutation.Transl Neurodegener. 2012 Jan 13;1(1):2. doi: 10.1186/2047-9158-1-2. Transl Neurodegener. 2012. PMID: 23210740 Free PMC article.
-
Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders.Sci Signal. 2015 May 12;8(376):ra45. doi: 10.1126/scisignal.2005965. Sci Signal. 2015. PMID: 25969543 Free PMC article.
-
The Neuroprotective Effect of Erythropoietin on Rotenone-Induced Neurotoxicity in SH-SY5Y Cells Through the Induction of Autophagy.Mol Neurobiol. 2016 Aug;53(6):3812-3821. doi: 10.1007/s12035-015-9316-x. Epub 2015 Jul 9. Mol Neurobiol. 2016. PMID: 26156288
References
-
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. - PubMed
-
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. - PubMed
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. - PubMed
-
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

