A key role for early growth response-1 and nuclear factor-kappaB in mediating and maintaining GRO/CXCR2 proliferative signaling in esophageal cancer
- PMID: 19435811
- PMCID: PMC6944287
- DOI: 10.1158/1541-7786.MCR-08-0472
A key role for early growth response-1 and nuclear factor-kappaB in mediating and maintaining GRO/CXCR2 proliferative signaling in esophageal cancer
Abstract
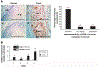
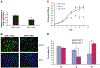
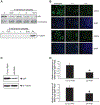
Although early growth response-1 (EGR-1) has been shown as a key transcription factor in controlling cell growth, proliferation, differentiation, and angiogenesis, its role in the development of esophageal cancer is poorly understood despite the high frequency of this disease in many parts of the world. Here, immunohistochemistry showed that EGR-1 is overexpressed in 80% of esophageal tumor tissues examined. Furthermore, EGR-1 is constitutively expressed in all esophageal cancer cell lines analyzed. Esophageal squamous carcinoma WHCO1 cells stably transfected with EGR-1 short hairpin RNA displayed a 55% reduction in EGR-1 protein levels, 50% reduction in cell proliferation, a 50% reduction in cyclin-dependent kinase 4 levels, and a 2-fold induction in p27(Kip1) levels associated with a G(2)-M cell cycle arrest. EGR-1 knockdown also caused a marked induction in IkappaBalpha expression, an effect also observed in GRObeta RNA interference-expressing WHCO1 cells, because EGR-1 lies downstream of GRO/CXCR2 signaling. Furthermore, p65 mRNA levels were also reduced in cells treated with either short hairpin RNA EGR-1 or small interfering RNA EGR-1. Immunohistochemical analysis indicated that p65 is elevated in 78% (n = 61) of esophageal tumor sections analyzed. Moreover, nuclear factor-kappaB inhibition with either sodium salicylate or p65 RNA interference led to a significant reduction in GROalpha and GRObeta expression. These results indicate that EGR-1 and nuclear factor-kappaB mediate GRO/CXCR2 proliferative signaling in esophageal cancer and may represent potential target molecules for therapeutic intervention.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures
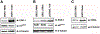

Similar articles
-
A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer.Cancer Res. 2006 Mar 15;66(6):3071-7. doi: 10.1158/0008-5472.CAN-05-2871. Cancer Res. 2006. PMID: 16540656
-
CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-κB activation via EGFR-transactivated Akt signaling.PLoS One. 2013 Dec 20;8(12):e83789. doi: 10.1371/journal.pone.0083789. eCollection 2013. PLoS One. 2013. PMID: 24376747 Free PMC article.
-
Progesterone and calcitriol attenuate inflammatory cytokines CXCL1 and CXCL2 in ovarian and endometrial cancer cells.J Cell Biochem. 2012 Oct;113(10):3143-52. doi: 10.1002/jcb.24191. J Cell Biochem. 2012. PMID: 22615136
-
Nuclear factor-kB signaling pathway constitutively activated in esophageal squamous cell carcinoma cell lines and inhibition of growth of cells by small interfering RNA.Acta Biochim Biophys Sin (Shanghai). 2006 May;38(5):318-26. doi: 10.1111/j.1745-7270.2006.00166.x. Acta Biochim Biophys Sin (Shanghai). 2006. PMID: 16680372
-
A role for CXCR2 in senescence, but what about in cancer?Cancer Res. 2009 Mar 15;69(6):2167-70. doi: 10.1158/0008-5472.CAN-08-3772. Epub 2009 Mar 10. Cancer Res. 2009. PMID: 19276354 Review.
Cited by
-
Clinical significance of serum expression of GROβ in esophageal squamous cell carcinoma.World J Gastroenterol. 2011 Jun 7;17(21):2658-62. doi: 10.3748/wjg.v17.i21.2658. World J Gastroenterol. 2011. PMID: 21677836 Free PMC article.
-
Overexpression of GRO-β is associated with an unfavorable outcome in colorectal cancer.Oncol Lett. 2016 Apr;11(4):2391-2397. doi: 10.3892/ol.2016.4222. Epub 2016 Feb 10. Oncol Lett. 2016. PMID: 27073485 Free PMC article.
-
Streptolysin S induces pronounced calcium-ion influx-dependent expression of immediate early genes encoding transcription factors.Sci Rep. 2023 Aug 22;13(1):13720. doi: 10.1038/s41598-023-40981-1. Sci Rep. 2023. PMID: 37608082 Free PMC article.
-
Triptolide-induced apoptosis by inactivating nuclear factor-kappa B apoptotic pathway in multiple myeloma in vitro.J Huazhong Univ Sci Technolog Med Sci. 2011 Aug;31(4):446. doi: 10.1007/s11596-011-0471-7. Epub 2011 Aug 7. J Huazhong Univ Sci Technolog Med Sci. 2011. PMID: 21823003
-
Chrysoeriol Prevents TNFα-Induced CYP19 Gene Expression via EGR-1 Downregulation in MCF7 Breast Cancer Cells.Int J Mol Sci. 2020 Oct 12;21(20):7523. doi: 10.3390/ijms21207523. Int J Mol Sci. 2020. PMID: 33053908 Free PMC article.
References
-
- Ebnet K, Vestweber D. Molecular mechanisms that control leukocyte extravasion: the selections and the chemokines. Histochem Cell Biol 1999;112:1–23. - PubMed
-
- Luan J, Shattuck-Brandt R, Haghnegahdar H, et al. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol 1997;62:588–97. - PubMed
-
- Norgauer J, Metzner B, Schraufstatter I. Expression and growth-promoting function of the IL-8 receptor β in human melanoma cells. J Immunol 1996; 156:1132–7. - PubMed
-
- Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Effects of MGSA/GROα on melanocyte transformation. Oncogene 1991;6:1115–24. - PubMed
-
- Owen JD, Strieter R, Burdick M, et al. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine β and γ proteins. Int J Cancer 1997;73:94–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

