Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3
- PMID: 19435806
- PMCID: PMC2684832
- DOI: 10.1242/jcs.040956
Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3
Abstract
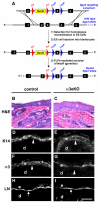
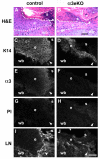
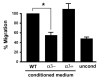
During cutaneous wound healing, epidermal keratinocytes play essential roles in the secretion of factors that promote angiogenesis. However, specific cues in the wound microenvironment that trigger the production of pro-angiogenic factors by keratinocytes, and the cellular receptors that mediate this response, remain unclear. In this study, we exploited a model of conditional integrin knockout to demonstrate impaired wound angiogenesis in mice that lack alpha3beta1 integrin in epidermis. In addition, we used genetic and shRNA approaches to determine that alpha3beta1-integrin deficiency in keratinocytes leads to reduced mRNA and protein expression of the pro-angiogenic factor mitogen-regulated protein 3 (MRP3; also known as PRL2C4), and to demonstrate that this regulation provides a mechanism of keratinocyte-to-endothelial-cell crosstalk that promotes endothelial-cell migration. Finally, we showed that the impaired wound angiogenesis in epidermis-specific alpha3-integrin-knockout mice is correlated with reduced expression of MRP3 in wounded epidermis. These findings identify a novel role for alpha3beta1 integrin in promoting wound angiogenesis through a mechanism of crosstalk from epidermal to endothelial cells, and they implicate MRP3 in this integrin-dependent crosstalk. Such a mechanism represents a novel paradigm for integrin-mediated regulation of wound angiogenesis that extends beyond traditional roles for integrins in cell adhesion and migration.
Figures

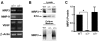

Similar articles
-
Suppression of integrin α3β1 by α9β1 in the epidermis controls the paracrine resolution of wound angiogenesis.J Cell Biol. 2017 May 1;216(5):1473-1488. doi: 10.1083/jcb.201510042. Epub 2017 Apr 17. J Cell Biol. 2017. PMID: 28416479 Free PMC article.
-
Keratinocyte integrin α3β1 induces expression of the macrophage stimulating factor, CSF-1, through a YAP/TEAD-dependent mechanism.Matrix Biol. 2024 Mar;127:48-56. doi: 10.1016/j.matbio.2024.02.003. Epub 2024 Feb 8. Matrix Biol. 2024. PMID: 38340968
-
Opposing Roles of Epidermal Integrins α3β1 and α9β1 in Regulation of mTLD/BMP-1-Mediated Laminin-γ2 Processing during Wound Healing.J Invest Dermatol. 2018 Feb;138(2):444-451. doi: 10.1016/j.jid.2017.09.004. Epub 2017 Sep 18. J Invest Dermatol. 2018. PMID: 28923241 Free PMC article.
-
Integrin-mediated regulation of epidermal wound functions.Cell Tissue Res. 2016 Sep;365(3):467-82. doi: 10.1007/s00441-016-2446-2. Epub 2016 Jun 28. Cell Tissue Res. 2016. PMID: 27351421 Free PMC article. Review.
-
Roles for epithelial integrin α3β1 in regulation of the microenvironment during normal and pathological tissue remodeling.Am J Physiol Cell Physiol. 2024 May 1;326(5):C1308-C1319. doi: 10.1152/ajpcell.00128.2024. Epub 2024 Mar 18. Am J Physiol Cell Physiol. 2024. PMID: 38497112 Review.
Cited by
-
Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing.EMBO Rep. 2010 Feb;11(2):97-105. doi: 10.1038/embor.2009.276. Epub 2010 Jan 15. EMBO Rep. 2010. PMID: 20075988 Free PMC article. Review.
-
The Yin and Yang of Integrin Function in Re-Epithelialization During Wound Healing.Adv Wound Care (New Rochelle). 2013 Apr;2(3):75-80. doi: 10.1089/wound.2011.0342. Adv Wound Care (New Rochelle). 2013. PMID: 24527329 Free PMC article. Review.
-
Decellularized matrix from tumorigenic human mesenchymal stem cells promotes neovascularization with galectin-1 dependent endothelial interaction.PLoS One. 2011;6(7):e21888. doi: 10.1371/journal.pone.0021888. Epub 2011 Jul 11. PLoS One. 2011. PMID: 21779348 Free PMC article.
-
Keratinocyte Function in Normal and Diabetic Wounds and Modulation by FOXO1.J Diabetes Res. 2020 Oct 28;2020:3714704. doi: 10.1155/2020/3714704. eCollection 2020. J Diabetes Res. 2020. PMID: 33195703 Free PMC article. Review.
-
α6β4 integrin, a master regulator of expression of integrins in human keratinocytes.J Biol Chem. 2012 May 25;287(22):17975-84. doi: 10.1074/jbc.M111.310458. Epub 2012 Apr 6. J Biol Chem. 2012. PMID: 22493440 Free PMC article.
References
-
- Bengtson, N. W. and Linzer, D. I. (2000). Inhibition of tumor growth by the antiangiogenic placental hormone, proliferin-related protein. Mol. Endocrinol. 14, 1934-1943. - PubMed
-
- Castilho, R. M., Squarize, C. H., Patel, V., Millar, S. E., Zheng, Y., Molinolo, A. and Gutkind, J. S. (2007). Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene 26, 5078-5085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

