Analysis of a large cluster of SLC22 transporter genes, including novel USTs, reveals species-specific amplification of subsets of family members
- PMID: 19417012
- PMCID: PMC2712220
- DOI: 10.1152/physiolgenomics.90309.2008
Analysis of a large cluster of SLC22 transporter genes, including novel USTs, reveals species-specific amplification of subsets of family members
Abstract
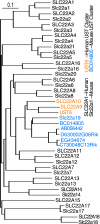
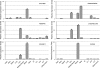
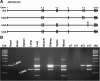
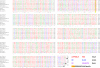
When the organic anion transporter Oat1 was first identified as NKT (Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. J Biol Chem 272: 6471-6478, 1997), it was argued that it, together with Oct1, may be part of a larger subfamily (now known as SLC22) involved in organic ion and xenobiotic transport. The least studied among SLC22 transporters are the so-called unknown substrate transporters (USTs). Here, five novel genes located in a cluster on mouse chromosome 19, immediately between Slc22a8 (Oat3)/Slc22a6 (Oat1) and Slc22a19 (Oat5), were identified as homologs of human USTs. These genes display preferential expression in liver and kidney, and one gene, AB056422, has several splicing variants with differential tissue expression and embryonic expression. Along with Slc22a6, Slc22a8, and Slc22a19, these Usts define the largest known cluster of mammalian Slc22 genes. Given the established functions of Oats, these genes may also be involved in organic anion transport. Usts have characteristic motifs and share a signature residue in the possible active site of transmembrane domain 7, a conserved, positively charged, amino acid, Arg356, possibly a site for interaction with organic anions. In certain species, Oat1 and Oat3 appeared to be highly conserved, whereas the Ust part of this cluster appeared to undergo repeated species-specific amplification, suggesting strong environmental selection pressure, and perhaps providing an explanation for copy number variation in the human locus. One Ust amplification in mouse appears to be recent. This cluster may be coordinately regulated and under selective pressure in a species-specific manner.
Figures



Similar articles
-
Evolutionary Analysis and Classification of OATs, OCTs, OCTNs, and Other SLC22 Transporters: Structure-Function Implications and Analysis of Sequence Motifs.PLoS One. 2015 Nov 4;10(11):e0140569. doi: 10.1371/journal.pone.0140569. eCollection 2015. PLoS One. 2015. PMID: 26536134 Free PMC article.
-
Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and Roct.Am J Physiol Renal Physiol. 2000 Apr;278(4):F635-43. doi: 10.1152/ajprenal.2000.278.4.F635. Am J Physiol Renal Physiol. 2000. PMID: 10751225
-
Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters.Physiol Genomics. 2004 Jun 17;18(1):12-24. doi: 10.1152/physiolgenomics.00014.2004. Epub 2004 Jun 17. Physiol Genomics. 2004. PMID: 15054140 Review.
-
Phylogenetic, syntenic, and tissue expression analysis of slc22 genes in zebrafish (Danio rerio).BMC Genomics. 2016 Aug 12;17(1):626. doi: 10.1186/s12864-016-2981-y. BMC Genomics. 2016. PMID: 27519738 Free PMC article.
-
The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease.Annu Rev Pharmacol Toxicol. 2018 Jan 6;58:663-687. doi: 10.1146/annurev-pharmtox-010617-052713. Annu Rev Pharmacol Toxicol. 2018. PMID: 29309257 Free PMC article. Review.
Cited by
-
Shared Ligands Between Organic Anion Transporters (OAT1 and OAT6) and Odorant Receptors.Drug Metab Dispos. 2015 Dec;43(12):1855-63. doi: 10.1124/dmd.115.065250. Epub 2015 Sep 10. Drug Metab Dispos. 2015. PMID: 26358290 Free PMC article.
-
Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis.Mol Pharmacol. 2009 Sep;76(3):481-90. doi: 10.1124/mol.109.056564. Epub 2009 Jun 10. Mol Pharmacol. 2009. PMID: 19515966 Free PMC article. Review.
-
Evolutionary Analysis and Classification of OATs, OCTs, OCTNs, and Other SLC22 Transporters: Structure-Function Implications and Analysis of Sequence Motifs.PLoS One. 2015 Nov 4;10(11):e0140569. doi: 10.1371/journal.pone.0140569. eCollection 2015. PLoS One. 2015. PMID: 26536134 Free PMC article.
-
Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1).J Proteome Res. 2011 Jun 3;10(6):2842-51. doi: 10.1021/pr200093w. Epub 2011 Apr 22. J Proteome Res. 2011. PMID: 21476605 Free PMC article.
-
Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney.Mol Pharmacol. 2011 Jul;80(1):147-54. doi: 10.1124/mol.110.070680. Epub 2011 Apr 14. Mol Pharmacol. 2011. PMID: 21493727 Free PMC article.
References
-
- Bailey TL, Baker ME, Elkan CP. An artificial intelligence approach to motif discovery in protein sequences: application to steriod dehydrogenases. J Steroid Biochem Mol Biol 62: 29–44, 1997. - PubMed
-
- Buist SC, Klaassen CD. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1-3; Slc22a6-8) mRNA levels. Drug Metab Dispos 32: 620–625, 2004. - PubMed
-
- Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol 146: 95–158, 2003. - PubMed
-
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417: 447–452, 2002. - PubMed
-
- Eraly SA, Hamilton BA, Nigam SK. Organic anion and cation transporters occur in pairs of similar and similarly expressed genes. Biochem Biophys Res Commun 300: 333–342, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

