Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS
- PMID: 19416874
- PMCID: PMC2675570
- DOI: 10.1073/pnas.0902505106
Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS
Abstract
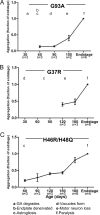
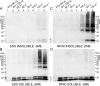
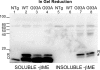
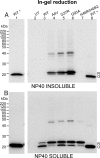
Transgenic mice that model familial (f)ALS, caused by mutations in superoxide dismutase (SOD)1, develop paralysis with pathology that includes the accumulation of aggregated forms of the mutant protein. Using a highly sensitive detergent extraction assay, we traced the appearance and abundance of detergent-insoluble and disulfide cross-linked aggregates of SOD1 throughout the disease course of SOD1-fALS mice (G93A, G37R, and H46R/H48Q). We demonstrate that the accumulation of disulfide cross-linked, detergent-insoluble, aggregates of mutant SOD1 occurs primarily in the later stages of the disease, concurrent with the appearance of rapidly progressing symptoms. We find no evidence for a model in which aberrant intermolecular disulfide bonding has an important role in promoting the aggregation of mutant SOD1, instead, such cross-linking appears to be a secondary event. Also, using both cell culture and mouse models, we find that mutant protein lacking the normal intramolecular disulfide bond is a major component of the insoluble SOD1 aggregates. Overall, our findings suggest a model in which soluble forms of mutant SOD1 initiate disease with larger aggregates implicated only in rapidly progressing events in the final stages of disease. Within the final stages of disease, abnormalities in the oxidation of a normal intramolecular disulfide bond in mutant SOD1 facilitate the aggregation of mutant protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis.J Biol Chem. 2008 May 16;283(20):13528-37. doi: 10.1074/jbc.M800564200. Epub 2008 Mar 3. J Biol Chem. 2008. PMID: 18316367 Free PMC article.
-
Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1.J Biol Chem. 2007 Sep 21;282(38):28087-95. doi: 10.1074/jbc.M704465200. Epub 2007 Jul 31. J Biol Chem. 2007. PMID: 17666395
-
Copper and zinc metallation status of copper-zinc superoxide dismutase from amyotrophic lateral sclerosis transgenic mice.J Biol Chem. 2011 Jan 28;286(4):2795-806. doi: 10.1074/jbc.M110.186999. Epub 2010 Nov 10. J Biol Chem. 2011. PMID: 21068388 Free PMC article.
-
Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation.Neuropathology. 2001 Mar;21(1):82-92. doi: 10.1046/j.1440-1789.2001.00361.x. Neuropathology. 2001. PMID: 11304046 Review.
-
SOD1 aggregation and ALS: role of metallation states and disulfide status.Curr Top Med Chem. 2012;12(22):2560-72. doi: 10.2174/1568026611212220010. Curr Top Med Chem. 2012. PMID: 23339308 Review.
Cited by
-
Discovery of Novel Inhibitors against ALS-Related SOD1(A4V) Aggregation through the Screening of a Chemical Library Using Differential Scanning Fluorimetry (DSF).Pharmaceuticals (Basel). 2024 Sep 27;17(10):1286. doi: 10.3390/ph17101286. Pharmaceuticals (Basel). 2024. PMID: 39458929 Free PMC article.
-
Mass spectrometry imaging of SOD1 protein-metal complexes in SOD1G93A transgenic mice implicates demetalation with pathology.Nat Commun. 2024 Aug 8;15(1):6518. doi: 10.1038/s41467-024-50514-7. Nat Commun. 2024. PMID: 39117623 Free PMC article.
-
Implications of ALS-Associated Mutations on Biochemical and Biophysical Features of hSOD1 and Aggregation Formation.Biochem Genet. 2024 Oct;62(5):3658-3680. doi: 10.1007/s10528-023-10619-y. Epub 2024 Jan 9. Biochem Genet. 2024. PMID: 38196030
-
Sympathetic neuropathology is revealed in muscles affected by amyotrophic lateral sclerosis.Front Physiol. 2023 May 12;14:1165811. doi: 10.3389/fphys.2023.1165811. eCollection 2023. Front Physiol. 2023. PMID: 37250128 Free PMC article.
-
Meiotic resetting of the cellular Sod1 pool is driven by protein aggregation, degradation, and transient LUTI-mediated repression.J Cell Biol. 2023 Mar 6;222(3):e202206058. doi: 10.1083/jcb.202206058. Epub 2023 Jan 9. J Cell Biol. 2023. PMID: 36622328 Free PMC article.
References
-
- Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Potter SZ, Valentine JS. The perplexing role of copper-zinc superoxide dismutase in amyotrophic lateral sclerosis (Lou Gehrig's disease) J Biol Inorg Chem. 2003;8:373–380. - PubMed
-
- Ratovitski T, et al. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Hum Mol Genet. 1999;8:1451–1460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

