Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo
- PMID: 19410272
- PMCID: PMC2743082
- DOI: 10.1016/j.virol.2009.04.001
Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo
Abstract
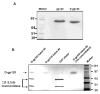
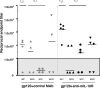
The Env glycoproteins gp120 and gp41 are used in humoral immunity-based vaccines against human immunodeficiency virus (HIV-1) infection. One among many obstacles to such a vaccine is the structural defenses of Env glycoproteins that limit their immunogenicity. For example, gp120 mannose residues can induce immunosuppressive responses in vitro, including IL-10 expression, via mannose C-type lectin receptors on antigen-presenting cells. Here, we have investigated whether mannose removal alters gp120 immunogenicity in mice. Administering demannosylated gp120 (D-gp120) in the T(H)2-skewing adjuvant Alum induced approximately 50-fold higher titers of anti-gp120 IgG, compared to unmodified gp120. While the IgG subclass profile was predominantly T(H)2-associated IgG1, Abs of the T(H)1-associated IgG2a and IgG3 subclasses were also detectable in D-gp120 recipients. Immunizing with D-gp120 also improved T-cell responses. Giving an IL-10 receptor blocking MAb together with unmodified gp120 in Alum increased the anti-gp120 IgG titer, implicating IL-10 as a possible mediator of auto-suppressive responses to gp120.
Figures



Similar articles
-
Occluding the mannose moieties on human immunodeficiency virus type 1 gp120 with griffithsin improves the antibody responses to both proteins in mice.AIDS Res Hum Retroviruses. 2012 Feb;28(2):206-14. doi: 10.1089/aid.2011.0101. Epub 2011 Jul 27. AIDS Res Hum Retroviruses. 2012. PMID: 21793733 Free PMC article.
-
Adsorption to aluminum hydroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 cytokine responses to HIV-1 gp120.J Immunol. 1997 Sep 1;159(5):2409-17. J Immunol. 1997. PMID: 9278332
-
Improving immunogenicity of HIV-1 envelope gp120 by glycan removal and immune complex formation.Vaccine. 2011 Nov 8;29(48):9064-74. doi: 10.1016/j.vaccine.2011.09.057. Epub 2011 Sep 23. Vaccine. 2011. PMID: 21945958 Free PMC article.
-
DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers.J Virol. 2015 Apr;89(8):4158-69. doi: 10.1128/JVI.02904-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631080 Free PMC article.
-
IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines.AIDS Res Hum Retroviruses. 2010 Apr;26(4):445-58. doi: 10.1089/aid.2009.0223. AIDS Res Hum Retroviruses. 2010. PMID: 20377426 Free PMC article.
Cited by
-
Glycan-dependent immunogenicity of recombinant soluble trimeric hemagglutinin.J Virol. 2012 Nov;86(21):11735-44. doi: 10.1128/JVI.01084-12. Epub 2012 Aug 22. J Virol. 2012. PMID: 22915811 Free PMC article.
-
Effects of adjuvants on IgG subclasses elicited by virus-like particles.J Transl Med. 2012 Jan 5;10:4. doi: 10.1186/1479-5876-10-4. J Transl Med. 2012. PMID: 22221900 Free PMC article.
-
Neutralizing Antibody Induction by HIV-1 Envelope Glycoprotein SOSIP Trimers on Iron Oxide Nanoparticles May Be Impaired by Mannose Binding Lectin.J Virol. 2020 Feb 28;94(6):e01883-19. doi: 10.1128/JVI.01883-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31852794 Free PMC article.
-
T-Cell Signaling in HIV-1 Infection.Open Virol J. 2013 Jul 26;7:57-71. doi: 10.2174/1874357920130621001. eCollection 2013. Open Virol J. 2013. PMID: 23986795 Free PMC article.
-
Lack of complex N-glycans on HIV-1 envelope glycoproteins preserves protein conformation and entry function.Virology. 2010 Jun 5;401(2):236-47. doi: 10.1016/j.virol.2010.02.019. Epub 2010 Mar 21. Virology. 2010. PMID: 20304457 Free PMC article.
References
-
- Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–62. - PubMed
-
- Allaway GP, Ryder AM, Beaudry GA, Maddon PJ. Synergistic inhibition of HIV-1 envelope-mediated cell fusion by CD4-based molecules in combination with antibodies to gp120 or gp41. AIDS Res Hum Retroviruses. 1993;9(7):581–7. - PubMed
-
- Barcova M, Kacani L, Speth C, Dierich MP. gp41 envelope protein of human immunodeficiency virus induces interleukin (IL)-10 in monocytes, but not in B, T, or NK cells, leading to reduced IL-2 and interferon-gamma production. J Infect Dis. 1998;177(4):905–13. - PubMed
-
- Beddows S, Schulke N, Kirschner M, Barnes K, Franti M, Michael E, Ketas T, Sanders RW, Maddon PJ, Olson WC, Moore JP. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2005;79(14):8812–27. - PMC - PubMed
-
- Bergman MP, Engering A, Smits HH, van Vliet SJ, van Bodegraven AA, Wirth HP, Kapsenberg ML, Vandenbroucke-Grauls CM, van Kooyk Y, Appelmelk BJ. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200(8):979–90. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

