Onset of human cytomegalovirus replication in fibroblasts requires the presence of an intact vimentin cytoskeleton
- PMID: 19403668
- PMCID: PMC2704777
- DOI: 10.1128/JVI.00398-09
Onset of human cytomegalovirus replication in fibroblasts requires the presence of an intact vimentin cytoskeleton
Abstract
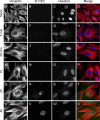
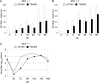
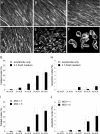
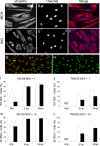
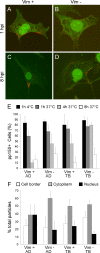
Like all viruses, herpesviruses extensively interact with the host cytoskeleton during entry. While microtubules and microfilaments appear to facilitate viral capsid transport toward the nucleus, evidence for a role of intermediate filaments in herpesvirus entry is lacking. Here, we examined the function of vimentin intermediate filaments in fibroblasts during the initial phase of infection of two genotypically distinct strains of human cytomegalovirus (CMV), one with narrow (AD169) and one with broad (TB40/E) cell tropism. Chemical disruption of the vimentin network with acrylamide, intermediate filament bundling in cells from a patient with giant axonal neuropathy, and absence of vimentin in fibroblasts from vimentin(-/-) mice severely reduced entry of either strain. In vimentin null cells, viral particles remained in the cytoplasm longer than in vimentin(+/+) cells. TB40/E infection was consistently slower than that of AD169 and was more negatively affected by the disruption or absence of vimentin. These findings demonstrate that an intact vimentin network is required for CMV infection onset, that intermediate filaments may function during viral entry to facilitate capsid trafficking and/or docking to the nuclear envelope, and that maintenance of a broader cell tropism is associated with a higher degree of dependence on the vimentin cytoskeleton.
Figures


Similar articles
-
Artemisinins target the intermediate filament protein vimentin for human cytomegalovirus inhibition.J Biol Chem. 2020 Oct 30;295(44):15013-15028. doi: 10.1074/jbc.RA120.014116. Epub 2020 Aug 27. J Biol Chem. 2020. PMID: 32855235 Free PMC article.
-
Impact of sequence variation in the UL128 locus on production of human cytomegalovirus in fibroblast and epithelial cells.J Virol. 2013 Oct;87(19):10489-500. doi: 10.1128/JVI.01546-13. Epub 2013 Jul 24. J Virol. 2013. PMID: 23885075 Free PMC article.
-
Cytoskeleton involvement during human cytomegalovirus replicative cycle in human embryo fibroblasts.New Microbiol. 2000 Jul;23(3):241-56. New Microbiol. 2000. PMID: 10939039
-
The diverse roles and dynamic rearrangement of vimentin during viral infection.J Cell Sci. 2020 Nov 5;134(5):jcs250597. doi: 10.1242/jcs.250597. J Cell Sci. 2020. PMID: 33154171 Review.
-
The Role of Host Cytoskeleton in Flavivirus Infection.Virol Sin. 2019 Feb;34(1):30-41. doi: 10.1007/s12250-019-00086-4. Epub 2019 Feb 6. Virol Sin. 2019. PMID: 30725318 Free PMC article. Review.
Cited by
-
Cell susceptibility to baculovirus transduction and echovirus infection is modified by protein kinase C phosphorylation and vimentin organization.J Virol. 2013 Sep;87(17):9822-35. doi: 10.1128/JVI.01004-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824807 Free PMC article.
-
Cellular Vimentin Interacts with Foot-and-Mouth Disease Virus Nonstructural Protein 3A and Negatively Modulates Viral Replication.J Virol. 2020 Jul 30;94(16):e00273-20. doi: 10.1128/JVI.00273-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32493819 Free PMC article.
-
Cytoskeletons in the Closet-Subversion in Alphaherpesvirus Infections.Viruses. 2018 Feb 13;10(2):79. doi: 10.3390/v10020079. Viruses. 2018. PMID: 29438303 Free PMC article. Review.
-
Acrylamide inhibits vaccinia virus through vimentin-independent anti-viral granule formation.Cell Microbiol. 2021 Aug;23(8):e13334. doi: 10.1111/cmi.13334. Epub 2021 May 3. Cell Microbiol. 2021. PMID: 33792166 Free PMC article.
-
The Microtubule Inhibitor Podofilox Inhibits an Early Entry Step of Human Cytomegalovirus.Viruses. 2016 Oct 24;8(10):295. doi: 10.3390/v8100295. Viruses. 2016. PMID: 27783035 Free PMC article.
References
-
- Adler, B., L. Scrivano, Z. Ruzcics, B. Rupp, C. Sinzger, and U. Koszinowski. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 872451-2460. - PubMed
-
- Aggeler, J., and K. Seely. 1990. Cytoskeletal dynamics in rabbit synovial fibroblasts. I. Effects of acrylamide on intermediate filaments and microfilaments. Cell Motil. Cytoskeleton 16110-120. - PubMed
-
- Akter, P., C. Cunningham, B. P. McSharry, A. Dolan, C. Addison, D. J. Dargan, A. F. Hassan-Walker, V. C. Emery, P. D. Griffiths, G. W. Wilkinson, and A. J. Davison. 2003. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 841117-1122. - PubMed
-
- Arcangeletti, M. C., F. Pinardi, M. C. Medici, E. Pilotti, F. De Conto, F. Ferraglia, M. P. Landini, C. Chezzi, and G. Dettori. 2000. Cytoskeleton involvement during human cytomegalovirus replicative cycle in human embryo fibroblasts. New Microbiol. 23241-256. - PubMed
-
- Belin, M. T., and P. Boulanger. 1985. Cytoskeletal proteins associated with intracytoplasmic human adenovirus at an early stage of infection. Exp. Cell Res. 160356-370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

