Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis
- PMID: 19400960
- PMCID: PMC2689857
- DOI: 10.1186/1742-2094-6-14
Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis
Abstract
Background: Interleukin-17A (IL-17A) is the founding member of a novel family of inflammatory cytokines that plays a critical role in the pathogenesis of many autoimmune diseases, including multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE). IL-17A signals through its receptor, IL-17RA, which is expressed in many peripheral tissues; however, expression of IL-17RA in the central nervous system (CNS) and its role in CNS inflammation are not well understood.
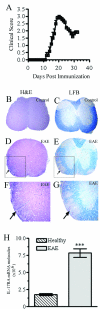
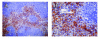
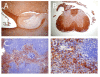
Methods: EAE was induced in C57Bl/6 mice by immunization with myelin oligodendroglial glycoprotein. IL-17RA expression in the CNS was compared between control and EAE mice using RT-PCR, in situ hybridization, and immunohistochemistry. Cell-type specific expression was examined in isolated astrocytic and microglial cell cultures. Cytokine and chemokine production was measured in IL-17A treated cultures to evaluate the functional status of IL-17RA.
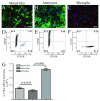
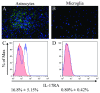
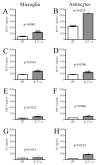
Results: Here we report increased IL-17RA expression in the CNS of mice with EAE, and constitutive expression of functional IL-17RA in mouse CNS tissue. Specifically, astrocytes and microglia express IL-17RA in vitro, and IL-17A treatment induces biological responses in these cells, including significant upregulation of MCP-1, MCP-5, MIP-2 and KC chemokine secretion. Exogenous IL-17A does not significantly alter the expression of IL-17RA in glial cells, suggesting that upregulation of chemokines by glial cells is due to IL-17A signaling through constitutively expressed IL-17RA.
Conclusion: IL-17RA expression is significantly increased in the CNS of mice with EAE compared to healthy mice, suggesting that IL-17RA signaling in glial cells can play an important role in autoimmune inflammation of the CNS and may be a potential pathway to target for therapeutic interventions.
Figures

Similar articles
-
Toll-like receptor signaling directly increases functional IL-17RA expression in neuroglial cells.Clin Immunol. 2014 Oct;154(2):127-40. doi: 10.1016/j.clim.2014.07.006. Epub 2014 Jul 27. Clin Immunol. 2014. PMID: 25076485
-
Diminished cytokine and chemokine expression in the central nervous system of GMF-deficient mice with experimental autoimmune encephalomyelitis.Brain Res. 2007 May 4;1144:239-47. doi: 10.1016/j.brainres.2007.01.075. Epub 2007 Jan 27. Brain Res. 2007. PMID: 17316572 Free PMC article.
-
Interleukin-36γ is expressed by neutrophils and can activate microglia, but has no role in experimental autoimmune encephalomyelitis.J Neuroinflammation. 2015 Sep 17;12:173. doi: 10.1186/s12974-015-0392-7. J Neuroinflammation. 2015. PMID: 26377915 Free PMC article.
-
IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease.Trends Immunol. 2011 May;32(5):232-9. doi: 10.1016/j.it.2011.02.007. Epub 2011 Apr 12. Trends Immunol. 2011. PMID: 21493143 Free PMC article. Review.
-
Recent advances in the IL-17 cytokine family.Curr Opin Immunol. 2011 Oct;23(5):613-9. doi: 10.1016/j.coi.2011.07.006. Epub 2011 Aug 16. Curr Opin Immunol. 2011. PMID: 21852080 Free PMC article. Review.
Cited by
-
Macrophages participate in IL-17-mediated inflammation.Eur J Immunol. 2012 Mar;42(3):726-36. doi: 10.1002/eji.201141737. Epub 2012 Jan 23. Eur J Immunol. 2012. PMID: 22161142 Free PMC article.
-
Human endometrial-derived mesenchymal stem cells suppress inflammation in the central nervous system of EAE mice.Stem Cell Rev Rep. 2012 Sep;8(3):940-52. doi: 10.1007/s12015-011-9338-3. Stem Cell Rev Rep. 2012. PMID: 22180029 No abstract available.
-
Inflammatory Cytokines Associated with Multiple Sclerosis Directly Induce Alterations of Neuronal Cytoarchitecture in Human Neurons.J Neuroimmune Pharmacol. 2023 Jun;18(1-2):145-159. doi: 10.1007/s11481-023-10059-w. Epub 2023 Mar 2. J Neuroimmune Pharmacol. 2023. PMID: 36862362 Free PMC article.
-
Haematopoietic stem cell gene therapy with IL-1Ra rescues cognitive loss in mucopolysaccharidosis IIIA.EMBO Mol Med. 2020 Mar 6;12(3):e11185. doi: 10.15252/emmm.201911185. Epub 2020 Feb 14. EMBO Mol Med. 2020. PMID: 32057196 Free PMC article.
-
Possible involvement of Interleukin-17A in the deterioration of prepulse inhibition on acoustic startle response in mice.Neuropsychopharmacol Rep. 2023 Sep;43(3):365-372. doi: 10.1002/npr2.12351. Epub 2023 Jun 6. Neuropsychopharmacol Rep. 2023. PMID: 37280178 Free PMC article.
References
-
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. - PubMed
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
-
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. - PubMed
-
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

