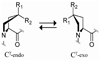
Modulating collagen triple-helix stability with 4-chloro, 4-fluoro, and 4-methylprolines
- PMID: 19400182
- PMCID: PMC2813564
- DOI: 10.1007/978-0-387-73657-0_115
Modulating collagen triple-helix stability with 4-chloro, 4-fluoro, and 4-methylprolines
Figures
Similar articles
-
4-ketoproline: An electrophilic proline analog for bioconjugation.Biopolymers. 2015 Mar;104(2):110-5. doi: 10.1002/bip.22620. Biopolymers. 2015. PMID: 25656588 Free PMC article.
-
Why Proline? Influence of Ring-Size on the Collagen Triple Helix.Org Lett. 2020 Jan 17;22(2):348-351. doi: 10.1021/acs.orglett.9b03528. Epub 2019 Nov 4. Org Lett. 2020. PMID: 31682124
-
Origin of the stability conferred upon collagen by fluorination.Bioorg Med Chem Lett. 2009 Jul 15;19(14):3859-62. doi: 10.1016/j.bmcl.2009.03.168. Epub 2009 Apr 21. Bioorg Med Chem Lett. 2009. PMID: 19423349 Free PMC article.
-
Insights on the conformational stability of collagen.Nat Prod Rep. 2002 Feb;19(1):49-59. doi: 10.1039/a903001h. Nat Prod Rep. 2002. PMID: 11902439 Review.
-
Recent progress on collagen triple helix structure, stability and assembly.Protein Pept Lett. 2002 Apr;9(2):107-16. doi: 10.2174/0929866023408922. Protein Pept Lett. 2002. PMID: 12141907 Review.
Cited by
-
(2S,4R)-4-Fluoro-pyrrolidinium-2-carboxyl-ate.Acta Crystallogr Sect E Struct Rep Online. 2012 Aug 1;68(Pt 8):o2490. doi: 10.1107/S1600536812031741. Epub 2012 Jul 18. Acta Crystallogr Sect E Struct Rep Online. 2012. PMID: 22904932 Free PMC article.
-
Interstrand dipole-dipole interactions can stabilize the collagen triple helix.J Biol Chem. 2011 Jul 1;286(26):22905-12. doi: 10.1074/jbc.M110.199984. Epub 2011 Apr 10. J Biol Chem. 2011. PMID: 21482820 Free PMC article.
-
Natural and Synthetic Halogenated Amino Acids-Structural and Bioactive Features in Antimicrobial Peptides and Peptidomimetics.Molecules. 2021 Dec 6;26(23):7401. doi: 10.3390/molecules26237401. Molecules. 2021. PMID: 34885985 Free PMC article. Review.
-
Synthesis of 5-fluoro- and 5-hydroxymethanoprolines via lithiation of N-BOC-methanopyrrolidines. Constrained Cγ-exo and Cγ-endo Flp and Hyp conformer mimics.J Org Chem. 2012 Jun 15;77(12):5331-44. doi: 10.1021/jo300700a. Epub 2012 May 25. J Org Chem. 2012. PMID: 22607128 Free PMC article.
-
An outcome-defining role for the triple-helical domain in regulating collagen-I assembly.Proc Natl Acad Sci U S A. 2024 Nov 12;121(46):e2412948121. doi: 10.1073/pnas.2412948121. Epub 2024 Nov 6. Proc Natl Acad Sci U S A. 2024. PMID: 39503893
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

