Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane
- PMID: 19398762
- PMCID: PMC2700393
- DOI: 10.1083/jcb.200811059
Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane
Abstract
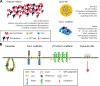
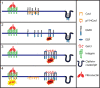
The plasma membrane is organized into various subdomains of clustered macromolecules. Such domains include adhesive structures (cellular synapses, substrate adhesions, and cell-cell junctions) and membrane invaginations (clathrin-coated pits and caveolae), as well as less well-defined domains such as lipid rafts and lectin-glycoprotein lattices. Domains are organized by specialized scaffold proteins including the intramembranous caveolins, which stabilize lipid raft domains, and the galectins, a family of animal lectins that cross-link glycoproteins forming molecular lattices. We review evidence that these heterogeneous microdomains interact to regulate substratum adhesion and cytokine receptor dynamics at the cell surface.
Figures
Similar articles
-
Epithelial cell-cell junctions and plasma membrane domains.Biochim Biophys Acta. 2009 Apr;1788(4):820-31. doi: 10.1016/j.bbamem.2008.07.015. Epub 2008 Jul 28. Biochim Biophys Acta. 2009. PMID: 18706883 Review.
-
Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling.Mol Pharmacol. 2009 Nov;76(5):1082-93. doi: 10.1124/mol.109.060160. Epub 2009 Aug 20. Mol Pharmacol. 2009. PMID: 19696145 Free PMC article.
-
Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains.Curr Opin Cell Biol. 1997 Aug;9(4):534-42. doi: 10.1016/s0955-0674(97)80030-0. Curr Opin Cell Biol. 1997. PMID: 9261060 Review.
-
Caveolin-1, galectin-3 and lipid raft domains in cancer cell signalling.Essays Biochem. 2015;57:189-201. doi: 10.1042/bse0570189. Essays Biochem. 2015. PMID: 25658354
-
GM1-containing lipid rafts are depleted within clathrin-coated pits.Curr Biol. 2003 Apr 15;13(8):686-90. doi: 10.1016/s0960-9822(03)00209-4. Curr Biol. 2003. PMID: 12699627
Cited by
-
The Enigmatic Role of Lipids in Cilia Signaling.Front Cell Dev Biol. 2020 Aug 11;8:777. doi: 10.3389/fcell.2020.00777. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850869 Free PMC article. Review.
-
Organizing the cell cortex: the role of ERM proteins.Nat Rev Mol Cell Biol. 2010 Apr;11(4):276-87. doi: 10.1038/nrm2866. Nat Rev Mol Cell Biol. 2010. PMID: 20308985 Free PMC article. Review.
-
Glycomics hits the big time.Cell. 2010 Nov 24;143(5):672-6. doi: 10.1016/j.cell.2010.11.008. Cell. 2010. PMID: 21111227 Free PMC article.
-
Magnesium Reduces Blood-Brain Barrier Permeability and Regulates Amyloid-β Transcytosis.Mol Neurobiol. 2018 Sep;55(9):7118-7131. doi: 10.1007/s12035-018-0896-0. Epub 2018 Jan 30. Mol Neurobiol. 2018. PMID: 29383689
-
Filming biomolecular processes by high-speed atomic force microscopy.Chem Rev. 2014 Mar 26;114(6):3120-88. doi: 10.1021/cr4003837. Epub 2014 Jan 30. Chem Rev. 2014. PMID: 24476364 Free PMC article. Review. No abstract available.
References
-
- Ahmad N., Gabius H.J., Andre S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C.F. 2004. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes.J. Biol. Chem. 279:10841–10847 - PubMed
-
- Chen I.J., Chen H.L., Demetriou M. 2007. Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling.J. Biol. Chem. 282:35361–35372 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

