Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A
- PMID: 19390585
- PMCID: PMC2669163
- DOI: 10.1371/journal.pone.0005254
Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A
Abstract
Background: This Phase 1/2a study evaluated the safety, immunogenicity, and efficacy of an experimental malaria vaccine comprised of the recombinant Plasmodium falciparum protein apical membrane antigen-1 (AMA-1) representing the 3D7 allele formulated with either the AS01B or AS02A Adjuvant Systems.
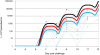
Methodology/principal findings: After a preliminary safety evaluation of low dose AMA-1/AS01B (10 microg/0.5 mL) in 5 adults, 30 malaria-naïve adults were randomly allocated to receive full dose (50 microg/0.5 mL) of AMA-1/AS01B (n = 15) or AMA-1/AS02A (n = 15), followed by a malaria challenge. All vaccinations were administered intramuscularly on a 0-, 1-, 2-month schedule. All volunteers experienced transient injection site erythema, swelling and pain. Two weeks post-third vaccination, anti-AMA-1 Geometric Mean Antibody Concentrations (GMCs) with 95% Confidence Intervals (CIs) were high: low dose AMA-1/AS01B 196 microg/mL (103-371 microg/mL), full dose AMA-1/AS01B 279 microg/mL (210-369 microg/mL) and full dose AMA-1/AS02A 216 microg/mL (169-276 microg/mL) with no significant difference among the 3 groups. The three vaccine formulations elicited equivalent functional antibody responses, as measured by growth inhibition assay (GIA), against homologous but not against heterologous (FVO) parasites as well as demonstrable interferon-gamma (IFN-gamma) responses. To assess efficacy, volunteers were challenged with P. falciparum-infected mosquitoes, and all became parasitemic, with no significant difference in the prepatent period by either light microscopy or quantitative polymerase chain reaction (qPCR). However, a small but significant reduction of parasitemia in the AMA-1/AS02A group was seen with a statistical model employing qPCR measurements.
Significance: All three vaccine formulations were found to be safe and highly immunogenic. These immune responses did not translate into significant vaccine efficacy in malaria-naïve adults employing a primary sporozoite challenge model, but encouragingly, estimation of parasite growth rates from qPCR data may suggest a partial biological effect of the vaccine. Further evaluation of the immunogenicity and efficacy of the AMA-1/AS02A formulation is ongoing in a malaria-experienced pediatric population in Mali.
Trial registration: www.clinicaltrials.gov NCT00385047.
Conflict of interest statement
Figures
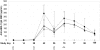
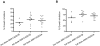


Similar articles
-
Phase I dose escalation safety and immunogenicity trial of Plasmodium falciparum apical membrane protein (AMA-1) FMP2.1, adjuvanted with AS02A, in malaria-naïve adults at the Walter Reed Army Institute of Research.Vaccine. 2007 May 22;25(21):4203-12. doi: 10.1016/j.vaccine.2007.03.012. Epub 2007 Mar 26. Vaccine. 2007. PMID: 17442466 Clinical Trial.
-
Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial.PLoS One. 2008 Jan 23;3(1):e1465. doi: 10.1371/journal.pone.0001465. PLoS One. 2008. PMID: 18213374 Free PMC article. Clinical Trial.
-
Preclinical evaluation of the safety and immunogenicity of a vaccine consisting of Plasmodium falciparum liver-stage antigen 1 with adjuvant AS01B administered alone or concurrently with the RTS,S/AS01B vaccine in rhesus primates.Infect Immun. 2008 Jan;76(1):229-38. doi: 10.1128/IAI.00977-07. Epub 2007 Oct 22. Infect Immun. 2008. PMID: 17954725 Free PMC article.
-
Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research.Vaccine. 2005 Mar 18;23(17-18):2243-50. doi: 10.1016/j.vaccine.2005.01.142. Vaccine. 2005. PMID: 15755604 Review.
-
Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review.Phytomedicine. 2019 Jul;60:152905. doi: 10.1016/j.phymed.2019.152905. Epub 2019 Mar 30. Phytomedicine. 2019. PMID: 31182297 Free PMC article. Review.
Cited by
-
ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans.Mol Ther. 2012 Dec;20(12):2355-68. doi: 10.1038/mt.2012.223. Epub 2012 Oct 23. Mol Ther. 2012. PMID: 23089736 Free PMC article. Clinical Trial.
-
A framework for in situ molecular characterization of coral holobionts using nanopore sequencing.Sci Rep. 2020 Sep 28;10(1):15893. doi: 10.1038/s41598-020-72589-0. Sci Rep. 2020. PMID: 32985530 Free PMC article.
-
Malaria invasion ligand RH5 and its prime candidacy in blood-stage malaria vaccine design.Hum Vaccin Immunother. 2015;11(6):1465-73. doi: 10.1080/21645515.2015.1026496. Hum Vaccin Immunother. 2015. PMID: 25844685 Free PMC article. Review.
-
A chimeric Plasmodium falciparum merozoite surface protein vaccine induces high titers of parasite growth inhibitory antibodies.Infect Immun. 2013 Oct;81(10):3843-54. doi: 10.1128/IAI.00522-13. Epub 2013 Jul 29. Infect Immun. 2013. PMID: 23897613 Free PMC article.
-
Bliss' and Loewe's additive and synergistic effects in Plasmodium falciparum growth inhibition by AMA1-RON2L, RH5, RIPR and CyRPA antibody combinations.Sci Rep. 2020 Jul 16;10(1):11802. doi: 10.1038/s41598-020-67877-8. Sci Rep. 2020. PMID: 32678144 Free PMC article.
References
-
- World Health Organization and UNICEF. World Malaria Report 2005. Report no. WHO/HTM/MAL/2005.1102. Geneva: World Health Organization; 2005. pp. 271–72.
-
- Duffy PE, Fried M. Malaria in the pregnant woman. Curr Top Microbiol Immun. 2005;295:169–200. - PubMed
-
- Moorthy VS, Good MF, Hill AVS. Malaria Vaccine Developments. Lancet. 2004;363:150–156. - PubMed
-
- Howell SA, Withers-Martinez C, Kocken CHM, Thomas AW, Blackman MJ. Proteolytic processing and primary structure of Plasmodium falciparum apical membrane antigen-1. J Biol Chem. 2001;276:31311–31320. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

