Polymerase-tailored variations in the water-mediated and substrate-assisted mechanism for nucleotidyl transfer: insights from a study of T7 DNA polymerase
- PMID: 19389406
- PMCID: PMC2801548
- DOI: 10.1016/j.jmb.2009.04.029
Polymerase-tailored variations in the water-mediated and substrate-assisted mechanism for nucleotidyl transfer: insights from a study of T7 DNA polymerase
Abstract
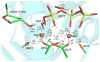
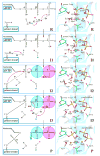
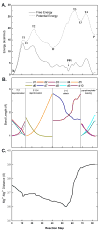
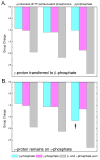
The nucleotidyl transfer reaction catalyzed by DNA polymerases is the critical step governing the accurate transfer of genetic information during DNA replication, and its malfunctioning can cause mutations leading to human diseases, including cancer. Here, utilizing ab initio quantum mechanical/molecular mechanical calculations with free-energy perturbation, we carried out an extensive investigation of the nucleotidyl transfer reaction mechanism in the well-characterized high-fidelity replicative DNA polymerase from phage T7. Our defined mechanism entails an initial concerted deprotonation of a conserved crystal water molecule with protonation of the gamma-phosphate of the deoxynucleotide triphosphate(dNTP) via a solvent water molecule, and then the proton on the primer 3'-terminus is transferred to the resulting hydroxide ion. Subsequently, the nucleophilic attack takes place, with the formation of a metastable pentacovalent phosphorane intermediate. Finally, the pyrophosphate leaves, facilitated by the relay of the proton on the gamma-phosphate to the alpha-beta bridging oxygen via solvent water. The computed activation free-energy barrier is consistent with kinetic data for the chemistry step with correct nucleotide incorporation in T7 DNA polymerase. This variant of the water-mediated and substrate-assisted mechanism has features tailored to the structure of the T7 DNA polymerase. However, a unifying theme in the water-mediated and substrate-assisted mechanism is the cycling through crystal and solvent water molecules of the proton originating from the primer 3'-terminus to the alpha-beta bridging oxygen of the deoxynucleotide triphosphate; this neutralizes the evolving negative charge as pyrophosphate leaves and restores the polymerase to its pre-chemistry state. These unifying features are likely requisite elements for nucleotidyl transfer reactions.
Figures
Similar articles
-
Preferred WMSA catalytic mechanism of the nucleotidyl transfer reaction in human DNA polymerase κ elucidates error-free bypass of a bulky DNA lesion.Nucleic Acids Res. 2012 Oct;40(18):9193-205. doi: 10.1093/nar/gks653. Epub 2012 Jul 5. Nucleic Acids Res. 2012. PMID: 22772988 Free PMC article.
-
Computer simulation of the chemical catalysis of DNA polymerases: discriminating between alternative nucleotide insertion mechanisms for T7 DNA polymerase.J Am Chem Soc. 2003 Jul 9;125(27):8163-77. doi: 10.1021/ja028997o. J Am Chem Soc. 2003. PMID: 12837086
-
A water-mediated and substrate-assisted catalytic mechanism for Sulfolobus solfataricus DNA polymerase IV.J Am Chem Soc. 2007 Apr 18;129(15):4731-7. doi: 10.1021/ja068821c. Epub 2007 Mar 22. J Am Chem Soc. 2007. PMID: 17375926 Free PMC article.
-
The mechanism of action of T7 DNA polymerase.Curr Opin Struct Biol. 1998 Dec;8(6):704-12. doi: 10.1016/s0959-440x(98)80089-4. Curr Opin Struct Biol. 1998. PMID: 9914251 Review.
-
Mechanism of the nucleotidyl-transfer reaction in DNA polymerase revealed by time-resolved protein crystallography.Biophysics (Nagoya-shi). 2013 Apr 20;9:31-6. doi: 10.2142/biophysics.9.31. eCollection 2013. Biophysics (Nagoya-shi). 2013. PMID: 27493538 Free PMC article. Review.
Cited by
-
Revelation of a catalytic calcium-binding site elucidates unusual metal dependence of a human apyrase.J Am Chem Soc. 2012 Sep 19;134(37):15595-603. doi: 10.1021/ja307267y. Epub 2012 Sep 10. J Am Chem Soc. 2012. PMID: 22928549 Free PMC article.
-
An overview of Y-Family DNA polymerases and a case study of human DNA polymerase η.Biochemistry. 2014 May 6;53(17):2793-803. doi: 10.1021/bi500019s. Epub 2014 Apr 23. Biochemistry. 2014. PMID: 24716551 Free PMC article. Review.
-
Effects of the Y432S Cancer-Associated Variant on the Reaction Mechanism of Human DNA Polymerase κ.J Chem Inf Model. 2024 May 27;64(10):4231-4249. doi: 10.1021/acs.jcim.4c00339. Epub 2024 May 8. J Chem Inf Model. 2024. PMID: 38717969
-
A new paradigm of DNA synthesis: three-metal-ion catalysis.Cell Biosci. 2016 Sep 6;6(1):51. doi: 10.1186/s13578-016-0118-2. eCollection 2016. Cell Biosci. 2016. PMID: 27602203 Free PMC article. Review.
-
Catalytic Mechanism of Mammalian Adenylyl Cyclase: A Computational Investigation.Biochemistry. 2015 Oct 13;54(40):6252-62. doi: 10.1021/acs.biochem.5b00655. Epub 2015 Oct 1. Biochemistry. 2015. PMID: 26393535 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

