Irreversibility of mitotic exit is the consequence of systems-level feedback
- PMID: 19387440
- PMCID: PMC2817895
- DOI: 10.1038/nature07984
Irreversibility of mitotic exit is the consequence of systems-level feedback
Abstract
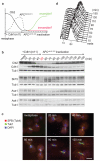
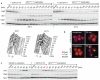
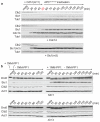
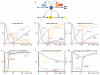
The eukaryotic cell cycle comprises an ordered series of events, orchestrated by the activity of cyclin-dependent kinases (Cdks), leading from chromosome replication during S phase to their segregation in mitosis. The unidirectionality of cell-cycle transitions is fundamental for the successful completion of this cycle. It is thought that irrevocable proteolytic degradation of key cell-cycle regulators makes cell-cycle transitions irreversible, thereby enforcing directionality. Here we have experimentally examined the contribution of cyclin proteolysis to the irreversibility of mitotic exit, the transition from high mitotic Cdk activity back to low activity in G1. We show that forced cyclin destruction in mitotic budding yeast cells efficiently drives mitotic exit events. However, these remain reversible after termination of cyclin proteolysis, with recovery of the mitotic state and cyclin levels. Mitotic exit becomes irreversible only after longer periods of cyclin degradation, owing to activation of a double-negative feedback loop involving the Cdk inhibitor Sic1 (refs 4, 5). Quantitative modelling suggests that feedback is required to maintain low Cdk activity and to prevent cyclin resynthesis. Our findings demonstrate that the unidirectionality of mitotic exit is not the consequence of proteolysis but of systems-level feedback required to maintain the cell cycle in a new stable state.
Figures
Similar articles
-
Cdc6 cooperates with Sic1 and Hct1 to inactivate mitotic cyclin-dependent kinases.Nature. 2001 Jul 19;412(6844):355-8. doi: 10.1038/35085610. Nature. 2001. PMID: 11460169
-
A quantitative model for ordered Cdk substrate dephosphorylation during mitotic exit.Cell. 2011 Nov 11;147(4):803-14. doi: 10.1016/j.cell.2011.09.047. Cell. 2011. PMID: 22078879
-
The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation.Mol Cell. 1998 Dec;2(6):709-18. doi: 10.1016/s1097-2765(00)80286-5. Mol Cell. 1998. PMID: 9885559
-
Phosphorylation and proteolysis: partners in the regulation of cell division in budding yeast.Curr Opin Genet Dev. 1997 Feb;7(1):7-16. doi: 10.1016/s0959-437x(97)80103-7. Curr Opin Genet Dev. 1997. PMID: 9024629 Review.
-
Cyclin destruction in mitosis: a crucial task of Cdc20.FEBS Lett. 2002 Dec 4;532(1-2):7-11. doi: 10.1016/s0014-5793(02)03657-8. FEBS Lett. 2002. PMID: 12459453 Review.
Cited by
-
Quantitative model of eukaryotic Cdk control through the Forkhead CONTROLLER.NPJ Syst Biol Appl. 2021 Jun 11;7(1):28. doi: 10.1038/s41540-021-00187-5. NPJ Syst Biol Appl. 2021. PMID: 34117265 Free PMC article. Review.
-
Design principles of the yeast G1/S switch.PLoS Biol. 2013 Oct;11(10):e1001673. doi: 10.1371/journal.pbio.1001673. Epub 2013 Oct 1. PLoS Biol. 2013. PMID: 24130459 Free PMC article.
-
Robust mitotic entry is ensured by a latching switch.Biol Open. 2013 Jul 26;2(9):924-31. doi: 10.1242/bio.20135199. eCollection 2013. Biol Open. 2013. PMID: 24143279 Free PMC article.
-
Measurement and modeling of transcriptional noise in the cell cycle regulatory network.Cell Cycle. 2013 Oct 1;12(19):3203-18. doi: 10.4161/cc.26257. Epub 2013 Sep 4. Cell Cycle. 2013. PMID: 24013422 Free PMC article.
-
Global Phosphoproteomic Mapping of Early Mitotic Exit in Human Cells Identifies Novel Substrate Dephosphorylation Motifs.Mol Cell Proteomics. 2015 Aug;14(8):2194-212. doi: 10.1074/mcp.M114.046938. Epub 2015 Jun 8. Mol Cell Proteomics. 2015. PMID: 26055452 Free PMC article.
References
-
- King RW, Deshaies RJ, Peters J-M, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. - PubMed
-
- Reed SI. Ratchets and clocks: the cell cycle, ubiquitylation and protein turnover. Nat. Rev. Mol. Cell Biol. 2003;4:855–864. - PubMed
-
- Donovan JD, Toyn JH, Johnson AL, Johnston LH. P40SDB25, a putative CDK inhibitor, has a role in the M/G1 transition in Saccharomyces cerevisiae. Genes Dev. 1994;8:1640–1653. - PubMed
-
- Visintin R, et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell. 1998;2:709–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

