Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia
- PMID: 19380227
- PMCID: PMC2859037
- DOI: 10.1016/j.nmd.2009.01.009
Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia
Abstract
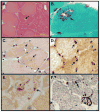
Mutations in valosin-containing protein (VCP) cause inclusion body myopathy (IBM) associated with Paget's disease of the bone (PDB) and fronto-temporal dementia (FTD) or IBMPFD. Although IBMPFD is a multisystem disorder, muscle weakness is the presenting symptom in greater than half of patients and an isolated symptom in 30%. Patients with the full spectrum of the disease make up only 12% of those affected; therefore it is important to consider and recognize IBMPFD in a neuromuscular clinic. The current review describes the skeletal muscle phenotype and common muscle histochemical features in IBMPFD. In addition to myopathic features; vacuolar changes and tubulofilamentous inclusions are found in a subset of patients. The most consistent findings are VCP, ubiquitin and TAR DNA-binding protein 43 (TDP-43) positive inclusions. VCP is a ubiquitously expressed multifunctional protein that is a member of the AAA+ (ATPase associated with various activities) protein family. It has been implicated in multiple cellular functions ranging from organelle biogenesis to protein degradation. Although the role of VCP in skeletal muscle is currently unknown, it is clear that VCP mutations lead to the accumulation of ubiquitinated inclusions and protein aggregates in patient tissue, transgenic animals and in vitro systems. We suggest that IBMPFD is novel type of protein surplus myopathy. Instead of accumulating a poorly degraded and aggregated mutant protein as seen in some myofibrillar and nemaline myopathies, VCP mutations disrupt its normal role in protein homeostasis resulting in the accumulation of ubiquitinated and aggregated proteins that are deleterious to skeletal muscle.
Figures

Similar articles
-
The multiple faces of valosin-containing protein-associated diseases: inclusion body myopathy with Paget's disease of bone, frontotemporal dementia, and amyotrophic lateral sclerosis.J Mol Neurosci. 2011 Nov;45(3):522-31. doi: 10.1007/s12031-011-9627-y. Epub 2011 Sep 3. J Mol Neurosci. 2011. PMID: 21892620 Review.
-
Valosin-containing protein and the pathogenesis of frontotemporal dementia associated with inclusion body myopathy.Acta Neuropathol. 2007 Jul;114(1):55-61. doi: 10.1007/s00401-007-0224-7. Epub 2007 Apr 25. Acta Neuropathol. 2007. PMID: 17457594 Review.
-
Valosin containing protein associated fronto-temporal lobar degeneration: clinical presentation, pathologic features and pathogenesis.Curr Alzheimer Res. 2011 May;8(3):252-60. doi: 10.2174/156720511795563773. Curr Alzheimer Res. 2011. PMID: 21222596 Free PMC article. Review.
-
Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation.Hum Mol Genet. 2006 Jan 15;15(2):189-99. doi: 10.1093/hmg/ddi426. Epub 2005 Dec 1. Hum Mol Genet. 2006. PMID: 16321991
-
Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease.J Cell Biol. 2009 Dec 14;187(6):875-88. doi: 10.1083/jcb.200908115. J Cell Biol. 2009. PMID: 20008565 Free PMC article.
Cited by
-
Is bRaQCing bad? New roles for ribosome associated quality control factors in stress granule regulation.Biochem Soc Trans. 2022 Dec 16;50(6):1715-1724. doi: 10.1042/BST20220549. Biochem Soc Trans. 2022. PMID: 36484689 Free PMC article.
-
Phosphorylated TDP-43 aggregates in skeletal and cardiac muscle are a marker of myogenic degeneration in amyotrophic lateral sclerosis and various conditions.Acta Neuropathol Commun. 2019 Oct 28;7(1):165. doi: 10.1186/s40478-019-0824-1. Acta Neuropathol Commun. 2019. PMID: 31661037 Free PMC article.
-
VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease.PLoS One. 2010 Oct 5;5(10):e13183. doi: 10.1371/journal.pone.0013183. PLoS One. 2010. PMID: 20957154 Free PMC article.
-
The updated retrospective questionnaire study of sporadic inclusion body myositis in Japan.Orphanet J Rare Dis. 2019 Jun 26;14(1):155. doi: 10.1186/s13023-019-1122-5. Orphanet J Rare Dis. 2019. PMID: 31242950 Free PMC article.
-
The multiple faces of valosin-containing protein-associated diseases: inclusion body myopathy with Paget's disease of bone, frontotemporal dementia, and amyotrophic lateral sclerosis.J Mol Neurosci. 2011 Nov;45(3):522-31. doi: 10.1007/s12031-011-9627-y. Epub 2011 Sep 3. J Mol Neurosci. 2011. PMID: 21892620 Review.
References
-
- Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–81. - PubMed
-
- Kimonis VE, Watts GD. Autosomal dominant inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Alzheimer Dis Assoc Disord. 2005;19 1:S44–7. - PubMed
-
- Guyant-Marechal L, Laquerriere A, Duyckaerts C, et al. Valosin-containing protein gene mutations: clinical and neuropathologic features. Neurology. 2006;67:644–51. - PubMed
-
- Haubenberger D, Bittner RE, Rauch-Shorny S, et al. Inclusion body myopathy and Paget disease is linked to a novel mutation in the VCP gene. Neurology. 2005;65:1304–5. - PubMed
-
- Hubbers CU, Clemen CS, Kesper K, et al. Pathological consequences of VCP mutations on human striated muscle. Brain. 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

