Alphavbeta3-targeted nanotherapy suppresses inflammatory arthritis in mice
- PMID: 19376816
- PMCID: PMC2735365
- DOI: 10.1096/fj.09-129874
Alphavbeta3-targeted nanotherapy suppresses inflammatory arthritis in mice
Abstract
The purpose of this study was to assess whether an alternative treatment approach that targets angiogenesis, delivered through ligand-targeted nanotherapy, would ameliorate inflammatory arthritis. Arthritis was induced using the K/BxN mouse model of inflammatory arthritis. After arthritis was clearly established, mice received three consecutive daily doses of alpha(v)beta(3)-targeted fumagillin nanoparticles. Control groups received no treatment or alpha(v)beta(3)-targeted nanoparticles without drugs. Disease score and paw thickness were measured daily. Mice that received alpha(v)beta(3)-targeted fumagillin nanoparticles showed a significantly lower disease activity score (mean score of 1.4+/-0.4; P<0.001) and change in ankle thickness (mean increase of 0.17+/-0.05 mm; P<0.001) 7 d after arthritis induction, whereas the group that received alpha(v)beta(3)-targeted nanoparticles without drugs exhibited a mean arthritic score of 9.0 +/- 0.3 and mean change in ankle thickness of 1.01 +/- 0.09 mm. Meanwhile, the group that received no treatment showed a mean arthritic score of 9.8 +/- 0.5 and mean change in ankle thickness of 1.05 +/- 0.10 mm. Synovial tissues from animals treated with targeted fumagillin nanoparticles also showed significant decrease in inflammation and angiogenesis and preserved proteoglycan integrity. Ligand-targeted nanotherapy to deliver antiangiogenic agents may represent an effective way to treat inflammatory arthritis.
Figures

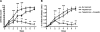
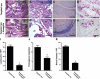

Similar articles
-
Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis.JACC Cardiovasc Imaging. 2008 Sep;1(5):624-34. doi: 10.1016/j.jcmg.2008.06.003. JACC Cardiovasc Imaging. 2008. PMID: 19356492 Free PMC article.
-
Three-dimensional MR mapping of angiogenesis with alpha5beta1(alpha nu beta3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model.FASEB J. 2008 Dec;22(12):4179-89. doi: 10.1096/fj.08-112060. Epub 2008 Aug 12. FASEB J. 2008. PMID: 18697838 Free PMC article.
-
Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis.Arterioscler Thromb Vasc Biol. 2006 Sep;26(9):2103-9. doi: 10.1161/01.ATV.0000235724.11299.76. Epub 2006 Jul 6. Arterioscler Thromb Vasc Biol. 2006. PMID: 16825592
-
Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis.Nat Clin Pract Rheumatol. 2007 Aug;3(8):434-42. doi: 10.1038/ncprheum0559. Nat Clin Pract Rheumatol. 2007. PMID: 17664950 Review.
-
Fumagillin: an anti-infective as a parent molecule for novel angiogenesis inhibitors.Expert Rev Anti Infect Ther. 2007 Aug;5(4):573-9. doi: 10.1586/14787210.5.4.573. Expert Rev Anti Infect Ther. 2007. PMID: 17678422 Review.
Cited by
-
Mechanisms and cell signaling in alcoholic liver disease.Biol Chem. 2010 Nov;391(11):1249-64. doi: 10.1515/BC.2010.137. Biol Chem. 2010. PMID: 20868231 Free PMC article. Review.
-
Conquering the dark side: colloidal iron oxide nanoparticles.ACS Nano. 2009 Dec 22;3(12):3917-26. doi: 10.1021/nn900819y. ACS Nano. 2009. PMID: 19908850 Free PMC article.
-
The application prospect of metal/metal oxide nanoparticles in the treatment of osteoarthritis.Naunyn Schmiedebergs Arch Pharmacol. 2021 Oct;394(10):1991-2002. doi: 10.1007/s00210-021-02131-0. Epub 2021 Aug 20. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 34415355 Free PMC article. Review.
-
Development of Nanomaterials to Target Articular Cartilage for Osteoarthritis Therapy.Front Mol Biosci. 2022 Aug 11;9:900344. doi: 10.3389/fmolb.2022.900344. eCollection 2022. Front Mol Biosci. 2022. PMID: 36032667 Free PMC article. Review.
-
Recent Advances in 19Fluorine Magnetic Resonance Imaging with Perfluorocarbon Emulsions.Engineering (Beijing). 2015 Dec;1(4):475-489. doi: 10.15302/J-ENG-2015103. Epub 2016 Mar 16. Engineering (Beijing). 2015. PMID: 27110430 Free PMC article.
References
-
- Gartlehner G, Hansen R A, Jonas B L, Thieda P, Lohr K N. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2006;33:2398–2408. - PubMed
-
- Zink A, Strangfeld A, Schneider M, Herzer P, Hierse F, Stoyanova-Scholz M, Wassenberg A, Kapelle A, Listing J. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006;54:3399–3407. - PubMed
-
- Siddiqui M A. The efficacy and tolerability of newer biologics in rheumatoid arthritis: best current evidence. Curr Opin Rheumatol. 2007;19:308–313s. - PubMed
-
- Buch M H, Bingham S J, Bryer D, Emery P. Long-term infliximab treatment in rheumatoid arthritis: subsequent outcome of initial responders. Rheumatology. 2007;46:1153–1156. - PubMed
-
- Tarner I H, Muller-Ladner U, Gay S. Emerging targets of biologic therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2007;3:336–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

