Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst
- PMID: 19372380
- PMCID: PMC2669790
- DOI: 10.1073/pnas.0902761106
Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst
Abstract
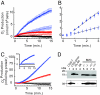
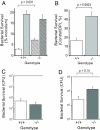
Granulocytes generate a "respiratory burst" of NADPH oxidase-dependent superoxide anion (O(2)(-*)) production that is required for efficient clearance of bacterial pathogens. Hv1 mediates a voltage-gated H(+) channel activity that is proposed to serve a charge-balancing role in granulocytic phagocytes such as neutrophils and eosinophils. Using mice in which the gene encoding Hv1 is replaced by beta-Geo reporter protein sequence, we show that Hv1 expression is required for measurable voltage-gated H(+) current in unstimulated phagocytes. O(2)(-*) production is substantially reduced in the absence of Hv1, suggesting that Hv1 contributes a majority of the charge compensation required for optimal NADPH oxidase activity. Despite significant reduction in superoxide production, Hv1(-/-) mice are able to clear several types of bacterial infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
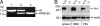

Similar articles
-
Molecular and functional characterization of Hv1 proton channel in human granulocytes.PLoS One. 2010 Nov 23;5(11):e14081. doi: 10.1371/journal.pone.0014081. PLoS One. 2010. PMID: 21124855 Free PMC article.
-
Do Hv1 proton channels regulate the ionic and redox homeostasis of phagosomes?Mol Cell Endocrinol. 2012 Apr 28;353(1-2):82-7. doi: 10.1016/j.mce.2011.10.005. Epub 2011 Oct 26. Mol Cell Endocrinol. 2012. PMID: 22056415 Review.
-
Comparison of proton channel, phagocyte oxidase, and respiratory burst levels between human eosinophil and neutrophil granulocytes.Free Radic Res. 2014 Oct;48(10):1190-9. doi: 10.3109/10715762.2014.938234. Epub 2014 Jul 21. Free Radic Res. 2014. PMID: 24985354
-
VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification.J Exp Med. 2010 Jan 18;207(1):129-39. doi: 10.1084/jem.20091837. Epub 2009 Dec 21. J Exp Med. 2010. PMID: 20026664 Free PMC article.
-
Regulation of Neutrophil Functions by Hv1/VSOP Voltage-Gated Proton Channels.Int J Mol Sci. 2021 Mar 5;22(5):2620. doi: 10.3390/ijms22052620. Int J Mol Sci. 2021. PMID: 33807711 Free PMC article. Review.
Cited by
-
Nicotine inhibits activation of microglial proton currents via interactions with α7 acetylcholine receptors.J Physiol Sci. 2017 Jan;67(1):235-245. doi: 10.1007/s12576-016-0460-5. Epub 2016 Jun 2. J Physiol Sci. 2017. PMID: 27256075 Free PMC article.
-
The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke.Nat Neurosci. 2012 Mar 4;15(4):565-73. doi: 10.1038/nn.3059. Nat Neurosci. 2012. PMID: 22388960 Free PMC article.
-
Inhibition of voltage-gated proton channels by local anaesthetics in GMI-R1 rat microglia.J Physiol. 2012 Feb 15;590(4):827-44. doi: 10.1113/jphysiol.2011.218149. Epub 2011 Dec 19. J Physiol. 2012. PMID: 22183729 Free PMC article.
-
Proton channel HVCN1 is required for effector functions of mouse eosinophils.BMC Immunol. 2013 May 24;14:24. doi: 10.1186/1471-2172-14-24. BMC Immunol. 2013. PMID: 23705768 Free PMC article.
-
Proton channels in non-phagocytic cells of the immune system.Wiley Interdiscip Rev Membr Transp Signal. 2013 Mar;2(2):65-73. doi: 10.1002/wmts.78. Wiley Interdiscip Rev Membr Transp Signal. 2013. PMID: 23710424 Free PMC article.
References
-
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. - PubMed
-
- Suenaga T, et al. Cloning of B cell-specific membrane tetraspanning molecule BTS possessing B cell proliferation-inhibitory function. Eur J Immunol. 2007;37:3197–3207. - PubMed
-
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. - PubMed
-
- DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

