Absence of nitric-oxide synthase in sequentially purified rat liver mitochondria
- PMID: 19372221
- PMCID: PMC2740410
- DOI: 10.1074/jbc.M109.003301
Absence of nitric-oxide synthase in sequentially purified rat liver mitochondria
Abstract
Data, both for and against the presence of a mitochondrial nitric-oxide synthase (NOS) isoform, is in the refereed literature. However, irrefutable evidence has not been forthcoming. In light of this controversy, we designed studies to investigate the existence of the putative mitochondrial NOS. Using repeated differential centrifugation followed by Percoll gradient fractionation, ultrapure, never frozen rat liver mitochondria and submitochondrial particles were obtained. Following trypsin digestion and desalting, the mitochondrial samples were analyzed by nano-HPLC-coupled linear ion trap-mass spectrometry. Linear ion trap-mass spectrometry analyses of rat liver mitochondria as well as submitochondrial particles were negative for any peptide from any NOS isoform. However, recombinant neuronal NOS-derived peptides from spiked mitochondrial samples were easily detected, down to 50 fmol on column. The protein calmodulin (CaM), absolutely required for NOS activity, was absent, whereas peptides from CaM-spiked samples were detected. Also, l-[(14)C]arginine to l-[(14)C]citrulline conversion assays were negative for NOS activity. Finally, Western blot analyses of rat liver mitochondria, using NOS (neuronal or endothelial) and CaM antibodies, were negative for any NOS isoform or CaM. In conclusion, and in light of our present limits of detection, data from carefully conducted, properly controlled experiments for NOS detection, utilizing three independent yet complementary methodologies, independently as well as collectively, refute the claim that a NOS isoform exists within rat liver mitochondria.
Figures

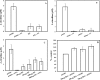
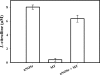
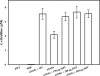
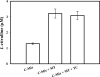

Similar articles
-
Arginase activity in mitochondria--An interfering factor in nitric oxide synthase activity assays.Biochem Biophys Res Commun. 2010 Apr 9;394(3):448-52. doi: 10.1016/j.bbrc.2009.10.169. Epub 2009 Nov 5. Biochem Biophys Res Commun. 2010. PMID: 19896461 Free PMC article.
-
Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS.Free Radic Biol Med. 2003 Nov 15;35(10):1217-28. doi: 10.1016/s0891-5849(03)00510-0. Free Radic Biol Med. 2003. PMID: 14607521
-
Purification and characterization of a nitric-oxide synthase from rat liver mitochondria.J Biol Chem. 1998 May 1;273(18):11044-8. doi: 10.1074/jbc.273.18.11044. J Biol Chem. 1998. PMID: 9556587
-
Arginine metabolism and the synthesis of nitric oxide in the nervous system.Prog Neurobiol. 2001 Jul;64(4):365-91. doi: 10.1016/s0301-0082(00)00056-3. Prog Neurobiol. 2001. PMID: 11275358 Review.
-
Alternative nitric oxide-producing substrates for NO synthases.Free Radic Biol Med. 2004 Oct 15;37(8):1105-21. doi: 10.1016/j.freeradbiomed.2004.06.031. Free Radic Biol Med. 2004. PMID: 15451052 Review.
Cited by
-
NO control of mitochondrial function in normal and transformed cells.Biochim Biophys Acta Bioenerg. 2017 Aug;1858(8):573-581. doi: 10.1016/j.bbabio.2017.02.009. Epub 2017 Feb 16. Biochim Biophys Acta Bioenerg. 2017. PMID: 28216426 Free PMC article. Review.
-
Mitochondrial thiols in the regulation of cell death pathways.Antioxid Redox Signal. 2012 Dec 15;17(12):1714-27. doi: 10.1089/ars.2012.4639. Epub 2012 Jun 11. Antioxid Redox Signal. 2012. PMID: 22530585 Free PMC article. Review.
-
Two strategies for the development of mitochondrion-targeted small molecule radiation damage mitigators.Int J Radiat Oncol Biol Phys. 2011 Jul 1;80(3):860-8. doi: 10.1016/j.ijrobp.2011.01.059. Epub 2011 Apr 13. Int J Radiat Oncol Biol Phys. 2011. PMID: 21493014 Free PMC article.
-
Regulation of mitochondrial processes by protein S-nitrosylation.Biochim Biophys Acta. 2012 Jun;1820(6):712-21. doi: 10.1016/j.bbagen.2011.03.008. Epub 2011 Mar 21. Biochim Biophys Acta. 2012. PMID: 21397666 Free PMC article. Review.
-
Arginase activity in mitochondria--An interfering factor in nitric oxide synthase activity assays.Biochem Biophys Res Commun. 2010 Apr 9;394(3):448-52. doi: 10.1016/j.bbrc.2009.10.169. Epub 2009 Nov 5. Biochem Biophys Res Commun. 2010. PMID: 19896461 Free PMC article.
References
-
- Ignarro L. J. (1989) FASEB J. 3, 31–36 - PubMed
-
- Shin W. S., Sasaki T., Kato M., Hara K., Seko A., Yang W. D., Shimamoto N., Sugimoto T., Toyo-oka T. (1992) J. Biol. Chem. 267, 20377–20382 - PubMed
-
- Malinski T., Taha Z., Grunfeld S., Patton S., Kapturczak M., Tomboulian P. (1993) Biochem. Biophys. Res. Commun. 193, 1076–1082 - PubMed
-
- Ignarro L. J., Buga G. M., Byrns R. E., Wood K. S., Chaudhuri G. (1988) J. Pharmacol. Exp. Ther. 246, 218–226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

