Estrogen, aging and the cardiovascular system
- PMID: 19371207
- PMCID: PMC3972065
- DOI: 10.2217/14796678.5.1.93
Estrogen, aging and the cardiovascular system
Abstract
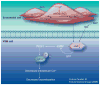
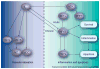
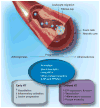
Estrogen is a powerful hormone with pleiotropic effects. Estrogens have potent antioxidant effects and are able to reduce inflammation, induce vasorelaxation and alter gene expression in both the vasculature and the heart. Estrogen treatment of cultured cardiac myocytes and endothelial cells rapidly activates NFkappaB, induces heat-shock protein (HSP)-72, a potent intracellular protective protein, and protects cells from simulated ischemia. In in vivo models, estrogens protect against ischemia and trauma/hemorrhage. Estrogens may decrease the expression of soluble epoxide hydrolase, which has deleterious effects on the cardiovascular system through metabolism of epoxyeicosatrienoic acids. Natural (endogenous) estrogens in premenopausal women appear to protect against cardiovascular disease and yet controlled clinical trials have not indicated a benefit from estrogen replacement postmenopause. Much remains to be understood in regards to the many properties of this powerful hormone and how changes in this hormone interact with aging-associated changes. The unexpected negative results of trials of estrogen replacement postmenopause probably arise from our lack of understanding of the many effects of this hormone.
Figures

Similar articles
-
Drug targeting of estrogen receptor signaling in the cardiovascular system: preclinical and clinical studies.Curr Med Chem Cardiovasc Hematol Agents. 2004 Apr;2(2):107-22. doi: 10.2174/1568016043477251. Curr Med Chem Cardiovasc Hematol Agents. 2004. PMID: 15320794 Review.
-
Sex hormone replacement therapy and modulation of vascular function in cardiovascular disease.Expert Rev Cardiovasc Ther. 2007 Jul;5(4):777-89. doi: 10.1586/14779072.5.4.777. Expert Rev Cardiovasc Ther. 2007. PMID: 17605655 Review.
-
Estrogens and the cardiovascular system: role of estradiol metabolites in hormone replacement therapy.Climacteric. 1998 Dec;1(4):296-301. doi: 10.3109/13697139809085558. Climacteric. 1998. PMID: 11907937 Review.
-
Sex steroids and vascular responses in hypertension and aging.Gend Med. 2008;5 Suppl A:S46-64. doi: 10.1016/j.genm.2008.03.006. Gend Med. 2008. PMID: 18395683 Review.
-
Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here?Hypertension. 2004 Dec;44(6):789-95. doi: 10.1161/01.HYP.0000145988.95551.28. Epub 2004 Oct 11. Hypertension. 2004. PMID: 15477384 Review.
Cited by
-
Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: translational implications.Front Neuroendocrinol. 2011 Aug;32(3):336-52. doi: 10.1016/j.yfrne.2010.12.005. Epub 2010 Dec 14. Front Neuroendocrinol. 2011. PMID: 21163293 Free PMC article. Review.
-
The significance of Brf1 overexpression in human hepatocellular carcinoma.Oncotarget. 2016 Feb 2;7(5):6243-54. doi: 10.18632/oncotarget.6668. Oncotarget. 2016. PMID: 26701855 Free PMC article.
-
Association of Bisphenol A Exposure With Hypertension and Early Macrovascular Diseases in Chinese Adults: A Cross-Sectional Study.Medicine (Baltimore). 2015 Oct;94(43):e1814. doi: 10.1097/MD.0000000000001814. Medicine (Baltimore). 2015. PMID: 26512580 Free PMC article.
-
Patterns of hospital admission with epistaxis for 26,725 patients over an 18-year period in Wales, UK.Ann R Coll Surg Engl. 2012 Nov;94(8):559-62. doi: 10.1308/003588412X13373405386691. Ann R Coll Surg Engl. 2012. PMID: 23131225 Free PMC article.
-
MicroRNA-23a participates in estrogen deficiency induced gap junction remodeling of rats by targeting GJA1.Int J Biol Sci. 2015 Feb 15;11(4):390-403. doi: 10.7150/ijbs.10930. eCollection 2015. Int J Biol Sci. 2015. PMID: 25798059 Free PMC article.
References
Bibliography
-
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. - PubMed
-
- Murphy E, Steenbergen C. Cardioprotection in females: a role for nitric oxide and altered gene expression. Heart Fail Rev. 2007;12:293–300. - PubMed
-
- Choudhry MA, Chaudry IH. 17β-estradiol: a novel hormone for improving immune and cardiovascular responses following trauma-hemorrhage. J Leukoc Biol. 2008;83:518–522. - PubMed
-
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. An insightful paper summarizing the beneficial effects of 17β-estradiol (E2) on the cardiovascular system. - PubMed
-
- Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol. 2003;95:2591–2597. - PubMed
Website
-
- American Association of Clinical Endocrinologists (AACE) position statement on hormone replacement therapy (HRT) and cardiovascular risk. 2008 www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_statement.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
