Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation
- PMID: 19366662
- PMCID: PMC2672544
- DOI: 10.1073/pnas.0809131106
Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation
Abstract
Enterohemorrhagic Escherichia coli O157:H7 translocates 2 effectors to trigger localized actin assembly in mammalian cells, resulting in filamentous actin "pedestals." One effector, the translocated intimin receptor (Tir), is localized in the plasma membrane and clustered upon binding the bacterial outer membrane protein intimin. The second, the proline-rich effector EspF(U) (aka TccP) activates the actin nucleation-promoting factor WASP/N-WASP, and is recruited to sites of bacterial attachment by a mechanism dependent on an Asn-Pro-Tyr (NPY(458)) sequence in the Tir C-terminal cytoplasmic domain. Tir, EspF(U), and N-WASP form a complex, but neither EspF(U) nor N-WASP bind Tir directly, suggesting involvement of another protein in complex formation. Screening of the mammalian SH3 proteome for the ability to bind EspF(U) identified the SH3 domain of insulin receptor tyrosine kinase substrate (IRTKS), a factor known to regulate the cytoskeleton. Derivatives of WASP, EspF(U), and the IRTKS SH3 domain were capable of forming a ternary complex in vitro, and replacement of the C terminus of Tir with the IRTKS SH3 domain resulted in a fusion protein competent for actin assembly in vivo. A second domain of IRTKS, the IRSp53/MIM homology domain (IMD), bound to Tir in a manner dependent on the C-terminal NPY(458) sequence, thereby recruiting IRTKS to sites of bacterial attachment. Ectopic expression of either the IRTKS SH3 domain or the IMD, or genetic depletion of IRTKS, blocked pedestal formation. Thus, enterohemorrhagic E. coli translocates 2 effectors that bind to distinct domains of a common host factor to promote the formation of a complex that triggers robust actin assembly at the plasma membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
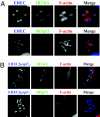
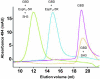

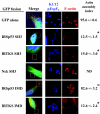

Comment in
-
Enterohemorrhagic Escherichia coli raises the I-BAR.Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6431-2. doi: 10.1073/pnas.0902773106. Epub 2009 Apr 20. Proc Natl Acad Sci U S A. 2009. PMID: 19380713 Free PMC article. No abstract available.
Similar articles
-
Enterohemorrhagic E. coli requires N-WASP for efficient type III translocation but not for EspFU-mediated actin pedestal formation.PLoS Pathog. 2010 Aug 19;6(8):e1001056. doi: 10.1371/journal.ppat.1001056. PLoS Pathog. 2010. PMID: 20808845 Free PMC article.
-
Nck adaptors, besides promoting N-WASP mediated actin-nucleation activity at pedestals, influence the cellular levels of enteropathogenic Escherichia coli Tir effector.Cell Adh Migr. 2014;8(4):404-17. doi: 10.4161/19336918.2014.969993. Cell Adh Migr. 2014. PMID: 25482634 Free PMC article.
-
Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly.PLoS Pathog. 2008 Oct;4(10):e1000191. doi: 10.1371/journal.ppat.1000191. Epub 2008 Oct 31. PLoS Pathog. 2008. PMID: 18974829 Free PMC article.
-
Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157:H7.Curr Opin Microbiol. 2003 Feb;6(1):82-90. doi: 10.1016/s1369-5274(03)00005-5. Curr Opin Microbiol. 2003. PMID: 12615225 Review.
-
Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal.Cell Microbiol. 2008 Mar;10(3):549-56. doi: 10.1111/j.1462-5822.2007.01103.x. Epub 2007 Dec 4. Cell Microbiol. 2008. PMID: 18053003 Review.
Cited by
-
EHEC Adhesins.Microbiol Spectr. 2014;2(2):EHEC00032013. doi: 10.1128/microbiolspec.EHEC-0003-2013. Microbiol Spectr. 2014. PMID: 25635238 Free PMC article.
-
Large-Scale Screening of Preferred Interactions of Human Src Homology-3 (SH3) Domains Using Native Target Proteins as Affinity Ligands.Mol Cell Proteomics. 2016 Oct;15(10):3270-3281. doi: 10.1074/mcp.M116.060483. Epub 2016 Jul 20. Mol Cell Proteomics. 2016. PMID: 27440912 Free PMC article.
-
Infection strategies of enteric pathogenic Escherichia coli.Gut Microbes. 2012 Mar-Apr;3(2):71-87. doi: 10.4161/gmic.19182. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22555463 Free PMC article. Review.
-
Pathogenic Escherichia coli Hijacks GTPase-Activated p21-Activated Kinase for Actin Pedestal Formation.mBio. 2019 Aug 20;10(4):e01876-19. doi: 10.1128/mBio.01876-19. mBio. 2019. PMID: 31431554 Free PMC article.
-
Enterohemorrhagic E. coli requires N-WASP for efficient type III translocation but not for EspFU-mediated actin pedestal formation.PLoS Pathog. 2010 Aug 19;6(8):e1001056. doi: 10.1371/journal.ppat.1001056. PLoS Pathog. 2010. PMID: 20808845 Free PMC article.
References
-
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. - PubMed
-
- Frankel G, Phillips AD. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell Microbiol. 2008;10:549–556. - PubMed
-
- Hayward RD, Leong JM, Koronakis V, Campellone KG. Exploiting pathogenic Escherichia coli to model transmembrane receptor signalling. Nat Rev Microbiol. 2006;4:358–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

