Neuropilin-1 identifies endothelial precursors in human and murine embryonic stem cells before CD34 expression
- PMID: 19364973
- PMCID: PMC2774135
- DOI: 10.1161/CIRCULATIONAHA.109.849596
Neuropilin-1 identifies endothelial precursors in human and murine embryonic stem cells before CD34 expression
Abstract
Background: In murine embryonic stem cells, the onset of vascular endothelial growth factor receptor 2 (VEGFR-2) expression identifies endothelial precursors. Undifferentiated human embryonic stem cells express VEGFR-2, and VEGFR-2 expression persists on differentiation. The objective of our study was to identify a single population of endothelial precursors with common identifying features from both human and murine embryonic stem cells.
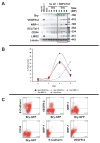
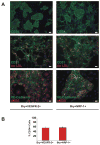
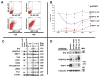
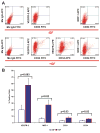
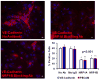
Methods and results: We report that expression of the VEGF coreceptor neuropilin-1 (NRP-1) coincides with expression of Brachyury and VEGFR-2 and identifies endothelial precursors in murine and human embryonic stem cells before CD31 or CD34 expression. When sorted and differentiated, VEGFR-2(+)NRP-1(+) cells form endothelial-like colonies that express CD31 and CD34 7-fold more efficiently than NRP-1 cells. Finally, antagonism of both the VEGF and Semaphorin binding functions of NRP-1 impairs the differentiation of vascular precursors to endothelial cells.
Conclusions: The onset of NRP-1 expression identifies endothelial precursors in murine and human stem cells. The findings define the origin of a single population of endothelial precursors from human and murine stem cells to endothelial cells. Additionally, the function of both the VEGF and Semaphorin binding activities of NRP-1 has important roles in the differentiation of stem cells to endothelial cells, providing novel insights into the role of NRP-1 in a model of vasculogenesis.
Figures

Similar articles
-
Exogenous recombinant dimeric neuropilin-1 is sufficient to drive angiogenesis.J Biol Chem. 2011 Jan 7;286(1):12-23. doi: 10.1074/jbc.M110.190801. Epub 2010 Oct 18. J Biol Chem. 2011. PMID: 20956519 Free PMC article.
-
Neuropilin-1 is required for endothelial cell adhesion to soluble vascular endothelial growth factor receptor 1.FEBS J. 2022 Jan;289(1):183-198. doi: 10.1111/febs.16119. Epub 2021 Aug 27. FEBS J. 2022. PMID: 34252269 Free PMC article.
-
Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells.Blood. 2008 Jan 1;111(1):122-31. doi: 10.1182/blood-2007-04-084186. Epub 2007 Sep 17. Blood. 2008. PMID: 17875805 Free PMC article.
-
[A novel molecular mechanism involving neuropilin-1 for vascular endothelial growth factor-induced retinal angiogenesis].Nippon Ganka Gakkai Zasshi. 2003 Nov;107(11):651-6. Nippon Ganka Gakkai Zasshi. 2003. PMID: 14661538 Review. Japanese.
-
Intracranial meningiomas, the VEGF-A pathway, and peritumoral brain oedema.Dan Med J. 2013 Apr;60(4):B4626. Dan Med J. 2013. PMID: 23651727 Review.
Cited by
-
Vascular tissue engineering: biodegradable scaffold platforms to promote angiogenesis.Stem Cell Res Ther. 2013 Jan 24;4(1):8. doi: 10.1186/scrt156. Stem Cell Res Ther. 2013. PMID: 23347554 Free PMC article. Review.
-
Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells.Nat Biotechnol. 2014 Nov;32(11):1151-1157. doi: 10.1038/nbt.3048. Epub 2014 Oct 12. Nat Biotechnol. 2014. PMID: 25306246 Free PMC article.
-
Neuropilin-1 regulates platelet-derived growth factor receptor signalling in mesenchymal stem cells.Biochem J. 2010 Mar 15;427(1):29-40. doi: 10.1042/BJ20091512. Biochem J. 2010. PMID: 20102335 Free PMC article.
-
The Nervous System Development Regulator Neuropilin-1 as a Potential Prognostic Marker and Therapeutic Target in Brain Cancer.Cancers (Basel). 2023 Oct 10;15(20):4922. doi: 10.3390/cancers15204922. Cancers (Basel). 2023. PMID: 37894289 Free PMC article. Review.
-
Neuropilin 1: function and therapeutic potential in cancer.Cancer Immunol Immunother. 2014 Feb;63(2):81-99. doi: 10.1007/s00262-013-1500-0. Epub 2013 Nov 22. Cancer Immunol Immunother. 2014. PMID: 24263240 Free PMC article. Review.
References
-
- Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69:73–82. - PubMed
-
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yuruqi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. - PubMed
-
- Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–27. - PubMed
-
- Sone M, Itoh I, Yamahara K, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, Nonoguchi A, Suzuki Y, Chao T-H, Sawada N, Fukunaga Y, Miyashita K, Park K, Oyamada N, Sawada N, Taura D, Tamura N, Kondo Y, Nito S, Suemori H, Nakatsuji N, Nishikawa S-I, Nakao K. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Atheroscler Thromb Vasc Biol. 2007;27:2127–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

