Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration
- PMID: 19363486
- PMCID: PMC2714540
- DOI: 10.1038/ncb1868
Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration
Abstract
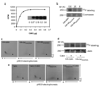
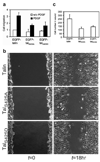
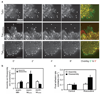
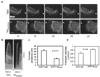
Cell migration is a dynamic process that requires temporal and spatial regulation of integrin activation and focal adhesion assembly/disassembly. Talin, an actin and beta-integrin tail-binding protein, is essential for integrin activation and focal adhesion formation. Calpain-mediated cleavage of talin has a key role in focal adhesion turnover; however, the talin head domain, one of the two cleavage products, stimulates integrin activation, localizes to focal adhesions and maintains cell edge protrusions, suggesting that other steps, downstream of talin proteolysis, are required for focal adhesion disassembly. Here we show that talin head binds Smurf1, an E3 ubiquitin ligase involved in cell polarity and migration, more tightly than full-length talin does and that this interaction leads to talin head ubiquitylation and degradation. We found that talin head is a substrate for Cdk5, a cyclin-dependent protein kinase that is essential for cell migration, synaptic transmission and cancer metastasis. Cdk5 phosphorylated talin head at Ser 425, inhibiting its binding to Smurf1, thus preventing talin head ubiquitylation and degradation. Expression of the mutant tal(S425A), which resists Cdk5 phosphorylation thereby increasing its susceptibility to Smurf1-mediated ubiqitylation, resulted in extensive focal adhesion turnover and inhibited cell migration. Thus, talin head produced by calpain-induced cleavage of talin is degraded through Smurf1-mediated ubiquitylation; moreover, phosphorylation by Cdk5 regulates the binding of Smurf1 to talin head, controlling talin head turnover, adhesion stability and ultimately, cell migration.
Figures
Similar articles
-
Roles of E3 ubiquitin ligases in cell adhesion and migration.Cell Adh Migr. 2010 Jan-Mar;4(1):10-8. doi: 10.4161/cam.4.1.9834. Epub 2010 Jan 18. Cell Adh Migr. 2010. PMID: 20009572 Free PMC article. Review.
-
Smurf1 zaps the talin head.Nat Cell Biol. 2009 May;11(5):538-40. doi: 10.1038/ncb0509-538. Nat Cell Biol. 2009. PMID: 19404335
-
Ubiquitylation of phosphatidylinositol 4-phosphate 5-kinase type I γ by HECTD1 regulates focal adhesion dynamics and cell migration.J Cell Sci. 2013 Jun 15;126(Pt 12):2617-28. doi: 10.1242/jcs.117044. Epub 2013 Apr 9. J Cell Sci. 2013. PMID: 23572508 Free PMC article.
-
Talin1 phosphorylation activates β1 integrins: a novel mechanism to promote prostate cancer bone metastasis.Oncogene. 2015 Apr 2;34(14):1811-21. doi: 10.1038/onc.2014.116. Epub 2014 May 5. Oncogene. 2015. PMID: 24793790 Free PMC article.
-
Tetraspanin CD9 in cell migration.Biochem Soc Trans. 2011 Apr;39(2):563-7. doi: 10.1042/BST0390563. Biochem Soc Trans. 2011. PMID: 21428940 Review.
Cited by
-
Deubiquitinase FAM/USP9X interacts with the E3 ubiquitin ligase SMURF1 protein and protects it from ligase activity-dependent self-degradation.J Biol Chem. 2013 Feb 1;288(5):2976-85. doi: 10.1074/jbc.M112.430066. Epub 2012 Nov 26. J Biol Chem. 2013. PMID: 23184937 Free PMC article.
-
The ubiquitin-proteasome system regulates focal adhesions at the leading edge of migrating cells.Elife. 2016 Sep 22;5:e17440. doi: 10.7554/eLife.17440. Elife. 2016. PMID: 27656905 Free PMC article.
-
Autophagy in adhesion and migration.J Cell Sci. 2016 Oct 15;129(20):3685-3693. doi: 10.1242/jcs.188490. Epub 2016 Sep 26. J Cell Sci. 2016. PMID: 27672021 Free PMC article. Review.
-
Roles of E3 ubiquitin ligases in cell adhesion and migration.Cell Adh Migr. 2010 Jan-Mar;4(1):10-8. doi: 10.4161/cam.4.1.9834. Epub 2010 Jan 18. Cell Adh Migr. 2010. PMID: 20009572 Free PMC article. Review.
-
Smurf1 regulates ameloblast polarization by ubiquitination-mediated degradation of RhoA.Cell Prolif. 2023 Apr;56(4):e13387. doi: 10.1111/cpr.13387. Epub 2022 Dec 29. Cell Prolif. 2023. PMID: 36579844 Free PMC article.
References
-
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells [mdash] over and over and over again. Nature Cell Biol. 2002;4:E97–E100. - PubMed
-
- Franco SJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nature Cell Biol. 2004;6:977–983. - PubMed
-
- Nuckolls GH, Romer LH, Burridge K. Microinjection of Antibodies against Talin Inhibits the Spreading and Migration of Fibroblasts. J. Cell Sci. 1992;102:753–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL057900-12A10002/HL/NHLBI NIH HHS/United States
- R01 AR027214/AR/NIAMS NIH HHS/United States
- U54 GM064346-089037/GM/NIGMS NIH HHS/United States
- P01 HL078784-050001/HL/NHLBI NIH HHS/United States
- U54 GM064346/GM/NIGMS NIH HHS/United States
- P01 HL031950-240012/HL/NHLBI NIH HHS/United States
- 1F32 HL08321/HL/NHLBI NIH HHS/United States
- P01 HL078784/HL/NHLBI NIH HHS/United States
- R01 AR027214-30/AR/NIAMS NIH HHS/United States
- P01 HL031950/HL/NHLBI NIH HHS/United States
- GM64346/GM/NIGMS NIH HHS/United States
- P01 HL057900/HL/NHLBI NIH HHS/United States
- F32 HL083215/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

