Characterization of Streptococcus gordonii SecA2 as a paralogue of SecA
- PMID: 19363114
- PMCID: PMC2681895
- DOI: 10.1128/JB.00365-09
Characterization of Streptococcus gordonii SecA2 as a paralogue of SecA
Abstract
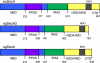
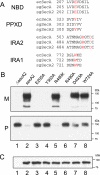
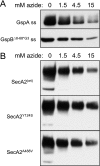
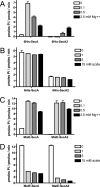
The accessory Sec system of Streptococcus gordonii is essential for transport of the glycoprotein GspB to the bacterial cell surface. A key component of this dedicated transport system is SecA2. The SecA2 proteins of streptococci and staphylococci are paralogues of SecA and are presumed to have an analogous role in protein transport, but they may be specifically adapted for the transport of large, serine-rich glycoproteins. We used a combination of genetic and biochemical methods to assess whether the S. gordonii SecA2 functions similarly to SecA. Although mutational analyses demonstrated that conserved amino acids are essential for the function of SecA2, replacing such residues in one of two nucleotide binding folds had only minor effects on SecA2 function. SecA2-mediated transport is highly sensitive to azide, as is SecA-mediated transport. Comparison of the S. gordonii SecA and SecA2 proteins in vitro revealed that SecA2 can hydrolyze ATP at a rate similar to that of SecA and is comparably sensitive to azide but that the biochemical properties of these enzymes are subtly different. That is, SecA2 has a lower solubility in aqueous solutions and requires higher Mg(2+) concentrations for maximal activity. In spite of the high degree of similarity between the S. gordonii paralogues, analysis of SecA-SecA2 chimeras indicates that the domains are not readily interchangeable. This suggests that specific, unique contacts between SecA2 and other components of the accessory Sec system may preclude cross-functioning with the canonical Sec system.
Figures
Similar articles
-
A Specific interaction between SecA2 and a region of the preprotein adjacent to the signal peptide occurs during transport via the accessory Sec system.J Biol Chem. 2012 Jul 13;287(29):24438-47. doi: 10.1074/jbc.M112.378059. Epub 2012 May 31. J Biol Chem. 2012. PMID: 22654116 Free PMC article.
-
Transport of preproteins by the accessory Sec system requires a specific domain adjacent to the signal peptide.J Bacteriol. 2010 Aug;192(16):4223-32. doi: 10.1128/JB.00373-10. Epub 2010 Jun 18. J Bacteriol. 2010. PMID: 20562303 Free PMC article.
-
An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets.Mol Microbiol. 2002 May;44(4):1081-94. doi: 10.1046/j.1365-2958.2002.02949.x. Mol Microbiol. 2002. PMID: 12010500
-
Selective transport by SecA2: an expanding family of customized motor proteins.Biochim Biophys Acta. 2014 Aug;1843(8):1674-86. doi: 10.1016/j.bbamcr.2013.10.019. Epub 2013 Oct 31. Biochim Biophys Acta. 2014. PMID: 24184206 Free PMC article. Review.
-
Emerging themes in SecA2-mediated protein export.Nat Rev Microbiol. 2012 Nov;10(11):779-89. doi: 10.1038/nrmicro2874. Epub 2012 Sep 24. Nat Rev Microbiol. 2012. PMID: 23000954 Free PMC article. Review.
Cited by
-
A role for glycosylated serine-rich repeat proteins in gram-positive bacterial pathogenesis.Mol Oral Microbiol. 2012 Aug;27(4):257-69. doi: 10.1111/j.2041-1014.2012.00653.x. Epub 2012 Jun 11. Mol Oral Microbiol. 2012. PMID: 22759311 Free PMC article. Review.
-
Differential localization of the streptococcal accessory sec components and implications for substrate export.J Bacteriol. 2013 Feb;195(4):682-95. doi: 10.1128/JB.01742-12. Epub 2012 Nov 30. J Bacteriol. 2013. PMID: 23204472 Free PMC article.
-
A Specific interaction between SecA2 and a region of the preprotein adjacent to the signal peptide occurs during transport via the accessory Sec system.J Biol Chem. 2012 Jul 13;287(29):24438-47. doi: 10.1074/jbc.M112.378059. Epub 2012 May 31. J Biol Chem. 2012. PMID: 22654116 Free PMC article.
-
The Two Distinct Types of SecA2-Dependent Export Systems.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.psib-0025-2018. doi: 10.1128/microbiolspec.PSIB-0025-2018. Microbiol Spectr. 2019. PMID: 31215505 Free PMC article. Review.
-
Streptococcus endopeptidases promote HPV infection in vitro.Microbiologyopen. 2019 Jan;8(1):e00628. doi: 10.1002/mbo3.628. Epub 2018 Apr 19. Microbiologyopen. 2019. PMID: 29675996 Free PMC article.
References
-
- Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 441081-1094. - PubMed
-
- Bensing, B. A., D. Takamatsu, and P. M. Sullam. 2005. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 581468-1481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

