Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein
- PMID: 19361478
- PMCID: PMC2670358
- DOI: 10.1016/j.neuroscience.2009.01.021
Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein
Abstract
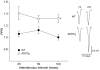
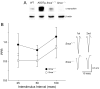
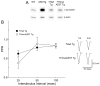
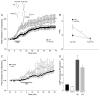
Most forms of Parkinson's disease (PD) are sporadic in nature, but some have genetic causes as first described for the alpha-synuclein gene. The alpha-synuclein protein also accumulates as insoluble aggregates in Lewy bodies in sporadic PD as well as in most inherited forms of PD. The focus of the present study is the modulation of synaptic plasticity in the corticostriatal pathway of transgenic (Tg) mice that overexpress the human alpha-synuclein protein throughout the brain (ASOTg). Paired-pulse facilitation was detected in vitro by activation of corticostriatal afferents in ASOTg mice, consistent with a presynaptic effect of elevated human alpha-synuclein. However basal synaptic transmission was unchanged in ASOTg, suggesting that human alpha-synuclein could impact paired-pulse facilitation via a presynaptic mechanism not directly related to the probability of neurotransmitter release. Mice lacking alpha-synuclein or those expressing normal and A53T human alpha-synuclein in tyrosine hydroxylase-containing neurons showed, instead, paired-pulse depression. High-frequency stimulation induced a presynaptic form of long-term depression solely in ASOTg striatum. A presynaptic, N-methyl-d-aspartate receptor-independent form of chemical long-term potentiation induced by forskolin (FSK) was enhanced in ASOTg striatum, while FSK-induced cAMP levels were reduced in ASOTg synaptoneurosome fractions. Overall the results suggest that elevated human alpha-synuclein alters presynaptic plasticity in the corticostriatal pathway, possibly reflecting a reduction in glutamate at corticostriatal synapses by modulation of adenylyl cyclase signaling pathways. ASOTg mice may recapitulate an early stage in PD during which overexpressed alpha-synuclein dampens corticostriatal synaptic transmission and reduces movement.
Figures
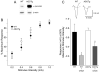
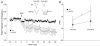
Similar articles
-
Alpha-synuclein overexpression in mice alters synaptic communication in the corticostriatal pathway.J Neurosci Res. 2010 Jun;88(8):1764-76. doi: 10.1002/jnr.22327. J Neurosci Res. 2010. PMID: 20029978 Free PMC article.
-
Altered long-term corticostriatal synaptic plasticity in transgenic mice overexpressing human CU/ZN superoxide dismutase (GLY(93)-->ALA) mutation.Neuroscience. 2003;118(2):399-408. doi: 10.1016/s0306-4522(02)00809-6. Neuroscience. 2003. PMID: 12699776
-
Aging and alpha-synuclein affect synaptic plasticity in the dentate gyrus.J Neural Transm (Vienna). 2009 Jan;116(1):13-22. doi: 10.1007/s00702-008-0149-x. Epub 2008 Nov 11. J Neural Transm (Vienna). 2009. PMID: 19002552
-
Synaptic dysfunction in Parkinson's disease.Adv Exp Med Biol. 2012;970:553-72. doi: 10.1007/978-3-7091-0932-8_24. Adv Exp Med Biol. 2012. PMID: 22351072 Review.
-
The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia.Trends Neurosci. 1996 Jan;19(1):19-24. doi: 10.1016/0166-2236(96)81862-5. Trends Neurosci. 1996. PMID: 8787136 Review.
Cited by
-
Inflammation, Infectious Triggers, and Parkinson's Disease.Front Neurol. 2019 Feb 19;10:122. doi: 10.3389/fneur.2019.00122. eCollection 2019. Front Neurol. 2019. PMID: 30837941 Free PMC article. Review.
-
Alpha synuclein post translational modifications: potential targets for Parkinson's disease therapy?Front Mol Neurosci. 2023 May 25;16:1197853. doi: 10.3389/fnmol.2023.1197853. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37305556 Free PMC article. Review.
-
Determining nuclear localization of alpha-synuclein in mouse brains.Neuroscience. 2011 Dec 29;199:318-32. doi: 10.1016/j.neuroscience.2011.10.016. Epub 2011 Oct 19. Neuroscience. 2011. PMID: 22033456 Free PMC article.
-
A critical window of CAG repeat-length correlates with phenotype severity in the R6/2 mouse model of Huntington's disease.J Neurophysiol. 2012 Jan;107(2):677-91. doi: 10.1152/jn.00762.2011. Epub 2011 Nov 9. J Neurophysiol. 2012. PMID: 22072510 Free PMC article.
-
A progressive mouse model of Parkinson's disease: the Thy1-aSyn ("Line 61") mice.Neurotherapeutics. 2012 Apr;9(2):297-314. doi: 10.1007/s13311-012-0104-2. Neurotherapeutics. 2012. PMID: 22350713 Free PMC article. Review.
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Akopian G, Musleh W, Smith R, Walsh JP. Functional state of corticostriatal synapses determines their expression of short- and long-term plasticity. Synapse. 2000;38:271–280. - PubMed
-
- Bamford NS, Zhang H, Schmitz Y, Wu N-P, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. - PubMed
-
- Berreta N, Nisticò, Bernardi G, Mercuri NB. Synaptic plasticity in the basal ganglia: A similar code for physiological and pathological conditions. Prog Neurobiol. 2008;84:343–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 NS038367-080002/NS/NINDS NIH HHS/United States
- NIH U54 ES012078/ES/NIEHS NIH HHS/United States
- P50 NS038367/NS/NINDS NIH HHS/United States
- NIH NS 38367/NS/NINDS NIH HHS/United States
- U54 ES012078-04/ES/NIEHS NIH HHS/United States
- P50 NS038367-070002/NS/NINDS NIH HHS/United States
- P50 NS038367-079005/NS/NINDS NIH HHS/United States
- U54 ES012078-03/ES/NIEHS NIH HHS/United States
- P50 NS038367-06A20002/NS/NINDS NIH HHS/United States
- P50 NS038367-089005/NS/NINDS NIH HHS/United States
- U54 ES012078/ES/NIEHS NIH HHS/United States
- P50 NS038367-06A29005/NS/NINDS NIH HHS/United States
- U54 ES012078-05/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

