Estradiol activates beta-catenin dependent transcription in neurons
- PMID: 19360103
- PMCID: PMC2664482
- DOI: 10.1371/journal.pone.0005153
Estradiol activates beta-catenin dependent transcription in neurons
Abstract
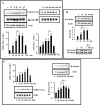
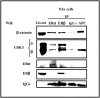
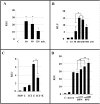
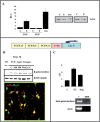
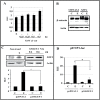
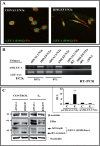
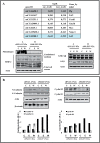
Estradiol may fulfill a plethora of functions in neurons, in which much of its activity is associated with its capacity to directly bind and dimerize estrogen receptors. This hormone-protein complex can either bind directly to estrogen response elements (ERE's) in gene promoters, or it may act as a cofactor at non-ERE sites interacting with other DNA-binding elements such as AP-1 or c-Jun. Many of the neuroprotective effects described for estrogen have been associated with this mode of action. However, recent evidence suggests that in addition to these "genomic effects", estrogen may also act as a more general "trophic factor" triggering cytoplasmic signals and extending the potential activity of this hormone. We demonstrated that estrogen receptor alpha associates with beta-catenin and glycogen synthase kinase 3 in the brain and in neurons, which has since been confirmed by others. Here, we show that the action of estradiol activates beta-catenin transcription in neuroblastoma cells and in primary cortical neurons. This activation is time and concentration-dependent, and it may be abolished by the estrogen receptor antagonist ICI 182780. The transcriptional activation of beta-catenin is dependent on lymphoid enhancer binding factor-1 (LEF-1) and a truncated-mutant of LEF-1 almost completely blocks estradiol TCF-mediated transcription. Transcription of a TCF-reporter in a transgenic mouse model is enhanced by estradiol in a similar fashion to that produced by Wnt3a. In addition, activation of a luciferase reporter driven by the engrailed promoter with three LEF-1 repeats was mediated by estradiol. We established a cell line that constitutively expresses a dominant-negative LEF-1 and it was used in a gene expression microarray analysis. In this way, genes that respond to estradiol or Wnt3a, sensitive to LEF-1, could be identified and validated. Together, these data demonstrate the existence of a new signaling pathway controlled by estradiol in neurons. This pathway shares some elements of the insulin-like growth factor-1/Insulin and Wnt signaling pathways, however, our data strongly suggest that it is different from that of both these ligands. These findings may reveal a set of new physiological roles for estrogens, at least in the Central Nervous System (CNS).
Conflict of interest statement
Figures


Similar articles
-
WNT3a Signaling Inhibits Aromatase Expression in Breast Adipose Fibroblasts-A Possible Mechanism Supporting the Loss of Estrogen Responsiveness of Triple-Negative Breast Cancers.Int J Mol Sci. 2023 Feb 28;24(5):4654. doi: 10.3390/ijms24054654. Int J Mol Sci. 2023. PMID: 36902090 Free PMC article.
-
Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter.J Biol Chem. 2002 Sep 6;277(36):33398-410. doi: 10.1074/jbc.M107977200. Epub 2002 Jun 6. J Biol Chem. 2002. PMID: 12052822
-
Progesterone initiates Wnt-beta-catenin signaling but estradiol is required for nuclear activation and synchronous proliferation of rat uterine stromal cells.J Endocrinol. 2006 Dec;191(3):537-48. doi: 10.1677/joe.1.07030. J Endocrinol. 2006. PMID: 17170212
-
Oestradiol regulates β-catenin-mediated transcription in neurones.J Neuroendocrinol. 2012 Jan;24(1):191-4. doi: 10.1111/j.1365-2826.2011.02186.x. J Neuroendocrinol. 2012. PMID: 21722217 Review.
-
Lymphoid enhancer factor/T cell factor expression in colorectal cancer.Cancer Metastasis Rev. 2004 Jan-Jun;23(1-2):41-52. doi: 10.1023/a:1025858928620. Cancer Metastasis Rev. 2004. PMID: 15000148 Review.
Cited by
-
GSK3β inhibition and canonical Wnt signaling in mice hearts after myocardial ischemic damage.PLoS One. 2019 Jun 20;14(6):e0218098. doi: 10.1371/journal.pone.0218098. eCollection 2019. PLoS One. 2019. PMID: 31220102 Free PMC article.
-
A cell-biased effect of estrogen in prion infection.J Virol. 2014 Jan;88(2):1342-53. doi: 10.1128/JVI.02936-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227845 Free PMC article.
-
Above genetics: lessons from cerebral development in autism.Transl Neurosci. 2011 Jun 1;2(2):106-120. doi: 10.2478/s13380-011-0016-3. Epub 2011 Jun 26. Transl Neurosci. 2011. PMID: 22523638 Free PMC article.
-
The Wnt/β-catenin signaling pathway is involved in the antitumor effect of fulvestrant on rat prolactinoma MMQ cells.Tumour Biol. 2014 Jun;35(6):5121-7. doi: 10.1007/s13277-013-1571-8. Epub 2014 Mar 19. Tumour Biol. 2014. PMID: 24643679
-
Dickkopf-1 blocks 17β-estradiol-enhanced object memory consolidation in ovariectomized female mice.Horm Behav. 2019 Aug;114:104545. doi: 10.1016/j.yhbeh.2019.06.009. Epub 2019 Aug 12. Horm Behav. 2019. PMID: 31228421 Free PMC article.
References
-
- Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogens on amyloid beta protein- induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem. 2001;(7):5287–5295. - PubMed
-
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. - PubMed
-
- Wise P. Estradiol exerts neuroprotective actions against ischemic brain injury: insights derived from animal models. Endocrine. 2003;21(1):11–15. - PubMed
-
- McKenna NJ, O'Malley BW. Nuclear receptors, coregulators, ligands, and selective receptor modulators: making sense of the patchwork quilt. Ann N Y Acad Sci. 2001;949:3–5. - PubMed
-
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83(6):851–857. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

