VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial
- PMID: 19352324
- PMCID: PMC2835194
- DOI: 10.1038/mt.2009.70
VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial
Abstract
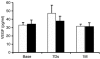
Despite the promise of proangiogenic gene therapy most clinical trials have failed to show benefit for the primary end point analysis. The NOGA angiogenesis Revascularization Therapy: assessment by RadioNuclide imaging (NORTHERN) trial was a double-blind, placebo-controlled study of intramyocardial vascular endothelial growth factor (VEGF165) gene therapy versus placebo, involving seven sites across Canada, designed to overcome major limitations of previous proangiogenic gene therapy trials. A total of 93 patients with refractory Canadian Cardiovascular Society (CCS) class 3 or 4 anginal symptoms were randomized to receive 2,000 microg of VEGF plasmid DNA or placebo (buffered saline) delivered via the endocardial route using an electroanatomical NOGA guidance catheter. There was no difference between the VEGF-treated and the placebo groups in the primary end point of change in myocardial perfusion from baseline to 3 or 6 months, assessed by single photon emission tomography (SPECT) imaging, although a significant reduction in the ischemic area was seen in both groups. Also, similar improvements in exercise treadmill time and anginal symptoms were seen in the VEGF and the placebo groups at 3 and 6 months, although again there were no differences between these groups. Despite the intramyocardial administration of a high "dose" of plasmid DNA using a percutaneous guidance catheter system, there was no benefit of VEGF gene therapy at 3 or 6 months for any of the end points studied.
Figures
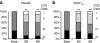
Similar articles
-
Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial.J Am Coll Cardiol. 2005 Apr 5;45(7):982-8. doi: 10.1016/j.jacc.2004.12.068. J Am Coll Cardiol. 2005. PMID: 15808751 Clinical Trial.
-
NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study.Circulation. 2005 Aug 30;112(9 Suppl):I157-65. doi: 10.1161/01.CIRCULATIONAHA.105.525782. Circulation. 2005. PMID: 16159809 Clinical Trial.
-
Intramyocardial plasmid-encoding human vascular endothelial growth factor A165/basic fibroblast growth factor therapy using percutaneous transcatheter approach in patients with refractory coronary artery disease (VIF-CAD).Am Heart J. 2011 Mar;161(3):581-9. doi: 10.1016/j.ahj.2010.11.023. Epub 2011 Jan 31. Am Heart J. 2011. PMID: 21392615 Clinical Trial.
-
Gene therapy for ischemic heart disease: therapeutic potential.Am J Cardiovasc Drugs. 2001;1(3):159-66. doi: 10.2165/00129784-200101030-00001. Am J Cardiovasc Drugs. 2001. PMID: 14728030 Review.
-
Therapeutic myocardial angiogenesis with vascular endothelial growth factors.Mol Cell Biochem. 2004 Sep;264(1-2):63-74. doi: 10.1023/b:mcbi.0000044375.33928.62. Mol Cell Biochem. 2004. PMID: 15544036 Review.
Cited by
-
Gene therapy to treat cardiovascular disease.J Am Heart Assoc. 2013 Aug 20;2(4):e000119. doi: 10.1161/JAHA.113.000119. J Am Heart Assoc. 2013. PMID: 23963752 Free PMC article. Review. No abstract available.
-
The Role of the Stem Cells Therapy in the Peripheral Artery Disease.Int J Mol Sci. 2019 May 7;20(9):2233. doi: 10.3390/ijms20092233. Int J Mol Sci. 2019. PMID: 31067647 Free PMC article. Review.
-
Comparison of Non-human Primate versus Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Treatment of Myocardial Infarction.Stem Cell Reports. 2018 Feb 13;10(2):422-435. doi: 10.1016/j.stemcr.2018.01.002. Epub 2018 Feb 1. Stem Cell Reports. 2018. PMID: 29398480 Free PMC article.
-
An optimal non-viral gene transfer method for genetically modifying porcine bone marrow-derived endothelial progenitor cells for experimental therapeutics.Sci Prog. 2021 Jul-Sep;104(3):368504211024113. doi: 10.1177/00368504211024113. Sci Prog. 2021. PMID: 34283683 Free PMC article.
-
Gene Therapy in Cardiac Surgery: Clinical Trials, Challenges, and Perspectives.Ann Thorac Surg. 2016 Jun;101(6):2407-16. doi: 10.1016/j.athoracsur.2015.12.004. Epub 2016 Jan 20. Ann Thorac Surg. 2016. PMID: 26801060 Free PMC article. Review.
References
-
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. - PubMed
-
- Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N.Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia Circulation 20021052012–2018.et al - PubMed
-
- Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR.Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA Ann Surg 1999230466–470.et al - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

