Nontoxic chemical interdiction of the epithelial-to-mesenchymal transition by targeting cap-dependent translation
- PMID: 19351181
- PMCID: PMC2796976
- DOI: 10.1021/cb9000475
Nontoxic chemical interdiction of the epithelial-to-mesenchymal transition by targeting cap-dependent translation
Abstract
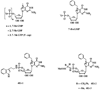
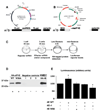
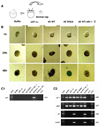
Normal growth and development depends upon high fidelity regulation of cap-dependent translation initiation, a process that is usurped and redirected in cancer to mediate acquisition of malignant properties. The epithelial-to-mesenchymal transition (EMT) is a key translationally regulated step in the development of epithelial cancers and pathological tissue fibrosis. To date, no compounds targeting EMT have been developed. Here we report the synthesis of a novel class of histidine triad nucleotide binding protein (HINT)-dependent pronucleotides that interdict EMT by negatively regulating the association of eIF4E with the mRNA cap. Compound eIF4E inhibitor-1 potently inhibited cap-dependent translation in a dose-dependent manner in zebrafish embryos without causing developmental abnormalities and prevented eIF4E from triggering EMT in zebrafish ectoderm explants without toxicity. Metabolism studies with whole cell lysates demonstrated that the prodrug was rapidly converted into 7-BnGMP. Thus we have successfully developed the first nontoxic small molecule able to inhibit EMT, a key process in the development of epithelial cancer and tissue fibrosis, by targeting the interaction of eIF4E with the mRNA cap and demonstrated the tractability of zebrafish as a model organism for studying agents that modulate EMT. Our work provides strong motivation for the continued development of compounds designed to normalize cap-dependent translation as novel chemo-preventive agents and therapeutics for cancer and fibrosis.
Figures


Similar articles
-
Treatment of breast and lung cancer cells with a N-7 benzyl guanosine monophosphate tryptamine phosphoramidate pronucleotide (4Ei-1) results in chemosensitization to gemcitabine and induced eIF4E proteasomal degradation.Mol Pharm. 2013 Feb 4;10(2):523-31. doi: 10.1021/mp300699d. Mol Pharm. 2013. PMID: 23289910 Free PMC article.
-
A novel inhibitor of cap-dependent translation initiation in yeast: p20 competes with eIF4G for binding to eIF4E.EMBO J. 1997 Mar 3;16(5):1114-21. doi: 10.1093/emboj/16.5.1114. EMBO J. 1997. PMID: 9118949 Free PMC article.
-
Transforming Growth Factor-β1 Induced Epithelial Mesenchymal Transition is blocked by a chemical antagonist of translation factor eIF4E.Sci Rep. 2015 Dec 18;5:18233. doi: 10.1038/srep18233. Sci Rep. 2015. PMID: 26678431 Free PMC article.
-
Cap-dependent translation initiation factor eIF4E: an emerging anticancer drug target.Med Res Rev. 2012 Jul;32(4):786-814. doi: 10.1002/med.21260. Epub 2012 Apr 11. Med Res Rev. 2012. PMID: 22495651 Free PMC article. Review.
-
Regulation of cap-dependent translation by eIF4E inhibitory proteins.Nature. 2005 Feb 3;433(7025):477-80. doi: 10.1038/nature03205. Nature. 2005. PMID: 15690031 Review.
Cited by
-
Development of a novel peptide aptamer that interacts with the eIF4E capped-mRNA binding site using peptide epitope linker evolution (PELE).RSC Chem Biol. 2022 May 19;3(7):916-930. doi: 10.1039/d2cb00099g. eCollection 2022 Jul 6. RSC Chem Biol. 2022. PMID: 35866173 Free PMC article.
-
Emergence of zebrafish models in oncology for validating novel anticancer drug targets and nanomaterials.Drug Discov Today. 2013 Feb;18(3-4):128-40. doi: 10.1016/j.drudis.2012.08.002. Epub 2012 Aug 10. Drug Discov Today. 2013. PMID: 22903142 Free PMC article. Review.
-
Migration and epithelial-to-mesenchymal transition of lung cancer can be targeted via translation initiation factors eIF4E and eIF4GI.Lab Invest. 2016 Sep;96(9):1004-15. doi: 10.1038/labinvest.2016.77. Epub 2016 Aug 8. Lab Invest. 2016. PMID: 27501049
-
Design, synthesis and evaluation of analogs of initiation factor 4E (eIF4E) cap-binding antagonist Bn7-GMP.Eur J Med Chem. 2010 Apr;45(4):1304-13. doi: 10.1016/j.ejmech.2009.11.054. Epub 2009 Dec 6. Eur J Med Chem. 2010. PMID: 20060622 Free PMC article.
-
Epithelial-mesenchymal transition: focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds.Mol Cancer. 2013 Sep 23;12(1):107. doi: 10.1186/1476-4598-12-107. Mol Cancer. 2013. PMID: 24053443 Free PMC article. Review.
References
-
- Gavert N, Ben-Ze'ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. - PubMed
-
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

