Identification of a Gene Cluster for the Biosynthesis of a Long, Galactose-Rich Exopolysaccharide in Lactobacillus rhamnosus GG and Functional Analysis of the Priming Glycosyltransferase
- PMID: 19346339
- PMCID: PMC2687306
- DOI: 10.1128/AEM.02919-08
Identification of a Gene Cluster for the Biosynthesis of a Long, Galactose-Rich Exopolysaccharide in Lactobacillus rhamnosus GG and Functional Analysis of the Priming Glycosyltransferase
Abstract
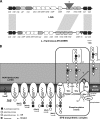
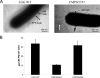
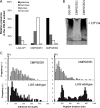
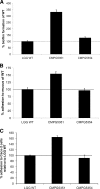
Cell surface polysaccharides have an established role as virulence factors in human bacterial pathogens. Less documented are the biosynthesis and biological functions of surface polysaccharides in beneficial bacteria. We identified a gene cluster that encodes the enzymes and regulatory and transporter proteins for the different steps in the biosynthesis of extracellular polysaccharides (EPS) of the well-documented probiotic strain Lactobacillus rhamnosus GG. Subsequent mutation of the welE gene, encoding the priming glycosyltransferase within this cluster, and comparative phenotypic analyses of wild-type versus mutant strains confirmed the specific function of this gene cluster in the biosynthesis of high-molecular-weight, galactose-rich heteropolymeric EPS molecules. The phenotypic analyses included monomer composition determination, estimation of the polymer length of the isolated EPS molecules, and single-molecule force spectroscopy of the surface polysaccharides. Further characterization of the welE mutant also showed that deprivation of these long, galactose-rich EPS molecules results in an increased adherence and biofilm formation capacity of L. rhamnosus GG, possibly because of less shielding of adhesins such as fimbria-like structures.
Figures
Similar articles
-
Functional analysis of luxS in the probiotic strain Lactobacillus rhamnosus GG reveals a central metabolic role important for growth and biofilm formation.J Bacteriol. 2007 Feb;189(3):860-71. doi: 10.1128/JB.01394-06. Epub 2006 Nov 10. J Bacteriol. 2007. PMID: 17098890 Free PMC article.
-
Characterization and complete genome sequences of L. rhamnosus DSM 14870 and L. gasseri DSM 14869 contained in the EcoVag® probiotic vaginal capsules.Microbiol Res. 2017 Dec;205:88-98. doi: 10.1016/j.micres.2017.08.003. Epub 2017 Aug 14. Microbiol Res. 2017. PMID: 28942850
-
Comparative analysis of the exopolysaccharide biosynthesis gene clusters from four strains of Lactobacillus rhamnosus.Microbiology (Reading). 2005 Jun;151(Pt 6):1839-1851. doi: 10.1099/mic.0.27852-0. Microbiology (Reading). 2005. PMID: 15941992
-
Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway.Can J Microbiol. 2014 Nov;60(11):697-716. doi: 10.1139/cjm-2014-0595. Epub 2014 Sep 16. Can J Microbiol. 2014. PMID: 25358682 Review.
-
Towards a better understanding of Lactobacillus rhamnosus GG--host interactions.Microb Cell Fact. 2014 Aug 29;13 Suppl 1(Suppl 1):S7. doi: 10.1186/1475-2859-13-S1-S7. Epub 2014 Aug 29. Microb Cell Fact. 2014. PMID: 25186587 Free PMC article. Review.
Cited by
-
Anti-Inflammatory Effect of an O-2-Substituted (1-3)-β-D-Glucan Produced by Pediococcus parvulus 2.6 in a Caco-2 PMA-THP-1 Co-Culture Model.Int J Mol Sci. 2022 Jan 28;23(3):1527. doi: 10.3390/ijms23031527. Int J Mol Sci. 2022. PMID: 35163449 Free PMC article.
-
Intracellular glycogen accumulation by human gut commensals as a niche adaptation trait.Gut Microbes. 2023 Jan-Dec;15(1):2235067. doi: 10.1080/19490976.2023.2235067. Gut Microbes. 2023. PMID: 37526383 Free PMC article. Review.
-
Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis.BMC Genomics. 2014 Dec 5;15:1070. doi: 10.1186/1471-2164-15-1070. BMC Genomics. 2014. PMID: 25480015 Free PMC article.
-
The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus.Antonie Van Leeuwenhoek. 2014 Oct;106(4):751-62. doi: 10.1007/s10482-014-0245-x. Epub 2014 Aug 5. Antonie Van Leeuwenhoek. 2014. PMID: 25090959 Free PMC article.
-
Gut health benefit and application of postbiotics in animal production.J Anim Sci Biotechnol. 2022 Apr 8;13(1):38. doi: 10.1186/s40104-022-00688-1. J Anim Sci Biotechnol. 2022. PMID: 35392985 Free PMC article. Review.
References
-
- Alvarez, M. A., M. Herrero, and J. E. Suarez. 1998. The site-specific recombination system of the Lactobacillus species bacteriophage A2 integrates in Gram-positive and Gram-negative bacteria. Virology 250:185-193. - PubMed
-
- Beg, A. M., M. N. Jones, T. Miller-Torbert, and R. G. Holt. 2002. Binding of Streptococcus mutans to extracellular matrix molecules and fibrinogen. Biochem. Biophys. Res. Commun. 298:75-79. - PubMed
-
- Bender, M. H., and J. Yother. 2001. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276:47966-47974. - PubMed
-
- Bentley, S. D., D. M. Aanensen, A. Mavroidi, D. Saunders, E. Rabbinowitsch, M. Collins, K. Donohoe, D. Harris, L. Murphy, M. A. Quail, G. Samuel, I. C. Skovsted, M. S. Kaltoft, B. Barrell, P. R. Reeves, J. Parkhill, and B. G. Spratt. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:262-269. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

