DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration
- PMID: 19344940
- PMCID: PMC2673108
- DOI: 10.1016/j.cell.2009.01.057
DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration
Abstract
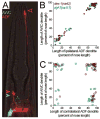
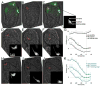
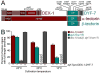
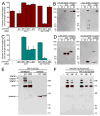
Cells are devices whose structures delimit function. For example, in the nervous system, neuronal and glial shapes dictate paths of information flow. To understand how cells acquire their shapes, we examined the formation of a sense organ in C. elegans. Using time-lapse imaging, we found that sensory dendrites form by stationary anchoring of dendritic tips during cell-body migration. A genetic screen identified DEX-1 and DYF-7, extracellular proteins required for dendritic tip anchoring, which act cooperatively at the time and place of anchoring. DEX-1 and DYF-7 contain, respectively, zonadhesin and zona pellucida domains, and DYF-7 self-associates into multimers important for anchoring. Thus, unlike other dendrites, amphid dendritic tips are positioned by DEX-1 and DYF-7 without the need for long-range guidance cues. In sequence and function, DEX-1 and DYF-7 resemble tectorins, which anchor stereocilia in the inner ear, suggesting that a sensory dendrite anchor may have evolved into part of a mechanosensor.
Figures


Comment in
-
Neurite extension: starting at the finish line.Cell. 2009 Apr 17;137(2):207-9. doi: 10.1016/j.cell.2009.04.001. Cell. 2009. PMID: 19379686 Free PMC article.
Similar articles
-
DYF-4 regulates patched-related/DAF-6-mediated sensory compartment formation in C. elegans.PLoS Genet. 2021 Jun 11;17(6):e1009618. doi: 10.1371/journal.pgen.1009618. eCollection 2021 Jun. PLoS Genet. 2021. PMID: 34115759 Free PMC article.
-
A multicellular rosette-mediated collective dendrite extension.Elife. 2019 Feb 15;8:e38065. doi: 10.7554/eLife.38065. Elife. 2019. PMID: 30767892 Free PMC article.
-
The molecular identities of the Caenorhabditis elegans intraflagellar transport genes dyf-6, daf-10 and osm-1.Genetics. 2006 Jul;173(3):1275-86. doi: 10.1534/genetics.106.056721. Epub 2006 Apr 30. Genetics. 2006. PMID: 16648645 Free PMC article.
-
Dendrite morphogenesis in Caenorhabditis elegans.Genetics. 2024 Jun 5;227(2):iyae056. doi: 10.1093/genetics/iyae056. Genetics. 2024. PMID: 38785371 Free PMC article. Review.
-
Assisted morphogenesis: glial control of dendrite shapes.Curr Opin Cell Biol. 2010 Oct;22(5):560-5. doi: 10.1016/j.ceb.2010.07.005. Epub 2010 Aug 2. Curr Opin Cell Biol. 2010. PMID: 20678911 Free PMC article. Review.
Cited by
-
DYF-4 regulates patched-related/DAF-6-mediated sensory compartment formation in C. elegans.PLoS Genet. 2021 Jun 11;17(6):e1009618. doi: 10.1371/journal.pgen.1009618. eCollection 2021 Jun. PLoS Genet. 2021. PMID: 34115759 Free PMC article.
-
C. elegans Apical Extracellular Matrices Shape Epithelia.J Dev Biol. 2020 Oct 6;8(4):23. doi: 10.3390/jdb8040023. J Dev Biol. 2020. PMID: 33036165 Free PMC article. Review.
-
Local microtubule organization promotes cargo transport in C. elegans dendrites.J Cell Sci. 2018 Oct 22;131(20):jcs223107. doi: 10.1242/jcs.223107. J Cell Sci. 2018. PMID: 30254025 Free PMC article.
-
Stereotyped behavioral maturation and rhythmic quiescence in C. elegans embryos.Elife. 2022 Aug 5;11:e76836. doi: 10.7554/eLife.76836. Elife. 2022. PMID: 35929725 Free PMC article.
-
A multicellular rosette-mediated collective dendrite extension.Elife. 2019 Feb 15;8:e38065. doi: 10.7554/eLife.38065. Elife. 2019. PMID: 30767892 Free PMC article.
References
-
- Altman J, Bayer SA. Vertical compartmentation and cellular transformations in the germinal matrices of the embryonic rat cerebral cortex. Exp Neurol. 1990;107:23–35. - PubMed
-
- Benard CY, Boyanov A, Hall DH, Hobert O. DIG-1, a novel giant protein, non-autonomously mediates maintenance of nervous system architecture. Development. 2006;133:3329–3340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

