LULL1 retargets TorsinA to the nuclear envelope revealing an activity that is impaired by the DYT1 dystonia mutation
- PMID: 19339278
- PMCID: PMC2688546
- DOI: 10.1091/mbc.e09-01-0094
LULL1 retargets TorsinA to the nuclear envelope revealing an activity that is impaired by the DYT1 dystonia mutation
Abstract
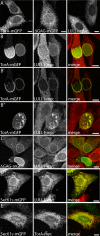
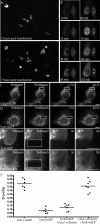
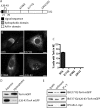
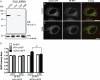
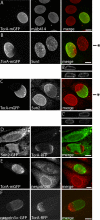
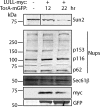
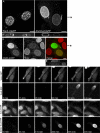
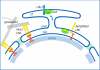
TorsinA (TorA) is an AAA+ ATPase in the endoplasmic reticulum (ER) lumen that is mutated in early onset DYT1 dystonia. TorA is an essential protein in mice and is thought to function in the nuclear envelope (NE) despite localizing throughout the ER. Here, we report that transient interaction of TorA with the ER membrane protein LULL1 targets TorA to the NE. FRAP and Blue Native PAGE indicate that TorA is a stable, slowly diffusing oligomer in either the absence or presence of LULL1. Increasing LULL1 expression redistributes both wild-type and disease-mutant TorA to the NE, while decreasing LULL1 with shRNAs eliminates intrinsic enrichment of disease-mutant TorA in the NE. When concentrated in the NE, TorA displaces the nuclear membrane proteins Sun2, nesprin-2G, and nesprin-3 while leaving nuclear pores and Sun1 unchanged. Wild-type TorA also induces changes in NE membrane structure. Because SUN proteins interact with nesprins to connect nucleus and cytoskeleton, these effects suggest a new role for TorA in modulating complexes that traverse the NE. Importantly, once concentrated in the NE, disease-mutant TorA displaces Sun2 with reduced efficiency and does not change NE membrane structure. Together, our data suggest that LULL1 regulates the distribution and activity of TorA within the ER and NE lumen and reveal functional defects in the mutant protein responsible for DYT1 dystonia.
Figures

Similar articles
-
The nuclear envelope localization of DYT1 dystonia torsinA-ΔE requires the SUN1 LINC complex component.BMC Cell Biol. 2011 May 31;12:24. doi: 10.1186/1471-2121-12-24. BMC Cell Biol. 2011. PMID: 21627841 Free PMC article.
-
Aberrant cellular behavior of mutant torsinA implicates nuclear envelope dysfunction in DYT1 dystonia.J Neurosci. 2004 Mar 17;24(11):2593-601. doi: 10.1523/JNEUROSCI.4461-03.2004. J Neurosci. 2004. PMID: 15028751 Free PMC article.
-
Access of torsinA to the inner nuclear membrane is activity dependent and regulated in the endoplasmic reticulum.J Cell Sci. 2015 Aug 1;128(15):2854-65. doi: 10.1242/jcs.167452. Epub 2015 Jun 19. J Cell Sci. 2015. PMID: 26092934 Free PMC article.
-
The Role of Torsin AAA+ Proteins in Preserving Nuclear Envelope Integrity and Safeguarding Against Disease.Biomolecules. 2020 Mar 19;10(3):468. doi: 10.3390/biom10030468. Biomolecules. 2020. PMID: 32204310 Free PMC article. Review.
-
Torsin ATPases: structural insights and functional perspectives.Curr Opin Cell Biol. 2016 Jun;40:1-7. doi: 10.1016/j.ceb.2016.01.001. Epub 2016 Jan 21. Curr Opin Cell Biol. 2016. PMID: 26803745 Free PMC article. Review.
Cited by
-
Dynamic regulation of hepatic lipid metabolism by torsinA and its activators.JCI Insight. 2024 Feb 8;9(3):e175328. doi: 10.1172/jci.insight.175328. JCI Insight. 2024. PMID: 38194265 Free PMC article.
-
DYT-TOR1A dystonia: an update on pathogenesis and treatment.Front Neurosci. 2023 Aug 10;17:1216929. doi: 10.3389/fnins.2023.1216929. eCollection 2023. Front Neurosci. 2023. PMID: 37638318 Free PMC article. Review.
-
TorsinA is essential for the timing and localization of neuronal nuclear pore complex biogenesis.bioRxiv [Preprint]. 2023 Apr 27:2023.04.26.538491. doi: 10.1101/2023.04.26.538491. bioRxiv. 2023. Update in: Nat Cell Biol. 2024 Sep;26(9):1482-1495. doi: 10.1038/s41556-024-01480-1. PMID: 37162852 Free PMC article. Updated. Preprint.
-
Genetic evidence of aberrant striatal synaptic maturation and secretory pathway alteration in a dystonia mouse model.Dystonia. 2022;1:10892. doi: 10.3389/dyst.2022.10892. Epub 2022 Dec 14. Dystonia. 2022. PMID: 36874764 Free PMC article.
-
Nuclear Morphological Abnormalities in Cancer: A Search for Unifying Mechanisms.Results Probl Cell Differ. 2022;70:443-467. doi: 10.1007/978-3-031-06573-6_16. Results Probl Cell Differ. 2022. PMID: 36348118 Free PMC article.
References
-
- Breakefield X. O., Blood A. J., Li Y., Hallett M., Hanson P. I., Standaert D. G. The pathophysiological basis of dystonias. Nat. Rev. Neurosci. 2008;9:222–234. - PubMed
-
- Crisp M., Burke B. The nuclear envelope as an integrator of nuclear and cytoplasmic architecture. FEBS Lett. 2008;582:2023–2032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

