Altered functional properties of satellite glial cells in compressed spinal ganglia
- PMID: 19330845
- PMCID: PMC2759416
- DOI: 10.1002/glia.20872
Altered functional properties of satellite glial cells in compressed spinal ganglia
Abstract
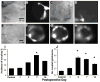
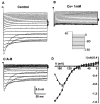
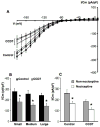
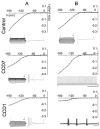
The cell bodies of sensory neurons in the dorsal root ganglion (DRG) are enveloped by satellite glial cells (SGCs). In an animal model of intervertebral foraminal stenosis and low-back pain, a chronic compression of the DRG (CCD) increases the excitability of neuronal cell bodies in the compressed ganglion. The morphological and electrophysiological properties of SGCs were investigated in both CCD and uninjured, control lumbar DRGs. SGCs responded within 12 h of the onset of CCD as indicated by an increased expression of glial fibrillary acidic protein (GFAP) in the compressed DRG but to lesser extent in neighboring or contralateral DRGs. Within 1 week, coupling through gap junctions between SGCs was significantly enhanced in the compressed ganglion. Under whole-cell patch clamp recordings, inward and outward potassium currents, but not sodium currents, were detected in individual SGCs. SGCs enveloping differently sized neurons had similar electrophysiological properties. SGCs in the compressed vs. control DRG exhibited significantly reduced inwardly rectifying potassium currents (Kir), increased input resistances and positively shifted resting membrane potentials. The reduction in Kir was greater for nociceptive medium-sized neurons compared to non-nociceptive neurons. Kir currents of SGCs around spontaneously active neurons were significantly reduced 1 day after compression but recovered by 7 days. These data demonstrate rapid alterations in glial membrane currents and GFAP expression in close temporal association with the development of neuronal hyperexcitability in the CCD model of neuropathic pain. However, these alterations are not fully sustained and suggest other mechanisms for the maintenance of the hyperexcitable state.
(c) 2009 Wiley-Liss, Inc.
Figures

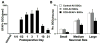

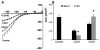
Similar articles
-
Chronic compression of mouse dorsal root ganglion alters voltage-gated sodium and potassium currents in medium-sized dorsal root ganglion neurons.J Neurophysiol. 2011 Dec;106(6):3067-72. doi: 10.1152/jn.00752.2011. Epub 2011 Sep 14. J Neurophysiol. 2011. PMID: 21917996 Free PMC article.
-
Enhanced excitability of dissociated primary sensory neurons after chronic compression of the dorsal root ganglion in the rat.Pain. 2005 Jan;113(1-2):106-12. doi: 10.1016/j.pain.2004.10.001. Pain. 2005. PMID: 15621370
-
Activation of GABA(B) receptors potentiates inward rectifying potassium currents in satellite glial cells from rat trigeminal ganglia: in vivo patch-clamp analysis.Neuroscience. 2015 Mar 12;288:51-8. doi: 10.1016/j.neuroscience.2014.12.024. Epub 2014 Dec 24. Neuroscience. 2015. PMID: 25542421
-
Role of satellite glial cells in gastrointestinal pain.Front Cell Neurosci. 2015 Oct 13;9:412. doi: 10.3389/fncel.2015.00412. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26528140 Free PMC article. Review.
-
2D <em>vs</em> 3D morphological analysis of dorsal root ganglia in health and painful neuropathy.Eur J Histochem. 2021 Oct 19;65(s1):3276. doi: 10.4081/ejh.2021.3276. Eur J Histochem. 2021. PMID: 34664808 Free PMC article. Review.
Cited by
-
Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy.Anesthesiology. 2014 Jun;120(6):1463-75. doi: 10.1097/ALN.0000000000000176. Anesthesiology. 2014. PMID: 24534904 Free PMC article.
-
Satellite glial cell proliferation in the trigeminal ganglia after chronic constriction injury of the infraorbital nerve.Glia. 2013 Dec;61(12):2000-8. doi: 10.1002/glia.22571. Epub 2013 Oct 3. Glia. 2013. PMID: 24123473 Free PMC article.
-
Canine dorsal root ganglia satellite glial cells represent an exceptional cell population with astrocytic and oligodendrocytic properties.Sci Rep. 2017 Oct 24;7(1):13915. doi: 10.1038/s41598-017-14246-7. Sci Rep. 2017. PMID: 29066783 Free PMC article.
-
Acute morphine activates satellite glial cells and up-regulates IL-1β in dorsal root ganglia in mice via matrix metalloprotease-9.Mol Pain. 2012 Mar 22;8:18. doi: 10.1186/1744-8069-8-18. Mol Pain. 2012. PMID: 22439811 Free PMC article.
-
Satellite glial GPR37L1 and its ligand maresin 1 regulate potassium channel signaling and pain homeostasis.J Clin Invest. 2024 Mar 26;134(9):e173537. doi: 10.1172/JCI173537. J Clin Invest. 2024. PMID: 38530364 Free PMC article.
References
-
- Anderson LC, von Bartheld CS, Byers MR. NGF depletion reduces ipsilateral and contralateral trigeminal satellite cell reactions after inferior alveolar nerve injury in adult rats. Exp Neurol. 1998;150:312–320. - PubMed
-
- Barres BA, Chun LL, Corey DP. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. - PubMed
-
- Barres BA. Glia ion channels. Curr Opin Neurobiol. 1991;1:354–359. - PubMed
-
- Bordey A, Lyons SA, Hablitz JJ, Sontheimer H. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J Neurophysiol. 2001;85:1719–1731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

