Worldwide distribution of HIV type 1 epitopes recognized by human anti-V3 monoclonal antibodies
- PMID: 19320565
- PMCID: PMC2853842
- DOI: 10.1089/aid.2008.0188
Worldwide distribution of HIV type 1 epitopes recognized by human anti-V3 monoclonal antibodies
Abstract
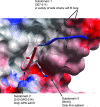
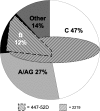
Epitopes, also known as antigenic determinants, are small clusters of specific atoms within macromolecules that are recognized by the immune system. Such epitopes can be targeted with vaccines designed to protect against specific pathogens. The third variable loop (V3 loop) of the HIV-1 pathogen's gp120 surface envelope glycoprotein can be a highly sensitive neutralization target. We derived sequence motifs for the V3 loop epitopes recognized by the human monoclonal antibodies (mAbs) 447-52D and 2219. Searching the HIV database for the occurrence of each epitope motif in worldwide viruses and correcting the results based on published WHO epidemiology reveal that the 447-52D epitope we defined occurs in 13% of viruses infecting patients worldwide: 79% of subtype B viruses, 1% of subtype C viruses, and 7% of subtype A/AG sequences. In contrast, the epitope we characterized for human anti-V3 mAb 2219 is present in 30% of worldwide isolates but is evenly distributed across the known HIV-1 subtypes: 48% of subtype B strains, 40% of subtype C, and 18% of subtype A/AG. Various assays confirmed that the epitopes corresponding to these motifs, when expressed in the SF162 Env backbone, were sensitively and specifically neutralized by the respective mAbs. The method described here is capable of accurately determining the worldwide occurrence and subtype distribution of any crystallographically resolved HIV-1 epitope recognized by a neutralizing antibody, which could be useful for multivalent vaccine design. More importantly, these calculations demonstrate that globally relevant, structurally conserved epitopes are present in the sequence variable V3 loop.
Figures


Similar articles
-
Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains.J Virol. 2005 Jun;79(11):6957-68. doi: 10.1128/JVI.79.11.6957-6968.2005. J Virol. 2005. PMID: 15890935 Free PMC article.
-
The cross-clade neutralizing activity of a human monoclonal antibody is determined by the GPGR V3 motif of HIV type 1.AIDS Res Hum Retroviruses. 2004 Nov;20(11):1254-8. doi: 10.1089/aid.2004.20.1254. AIDS Res Hum Retroviruses. 2004. PMID: 15588347
-
Design of immunogens that present the crown of the HIV-1 V3 loop in a conformation competent to generate 447-52D-like antibodies.Biochem J. 2006 Nov 1;399(3):483-91. doi: 10.1042/BJ20060588. Biochem J. 2006. PMID: 16827663 Free PMC article.
-
Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design.Nat Rev Immunol. 2010 Jul;10(7):527-35. doi: 10.1038/nri2801. Nat Rev Immunol. 2010. PMID: 20577269 Free PMC article. Review.
-
Max Bergmann lecture protein epitope mimetics in the age of structural vaccinology.J Pept Sci. 2013 Mar;19(3):127-40. doi: 10.1002/psc.2482. Epub 2013 Jan 24. J Pept Sci. 2013. PMID: 23349031 Free PMC article. Review.
Cited by
-
Resistance of Subtype C HIV-1 Strains to Anti-V3 Loop Antibodies.Adv Virol. 2012;2012:803535. doi: 10.1155/2012/803535. Epub 2012 Apr 2. Adv Virol. 2012. PMID: 22548061 Free PMC article.
-
Comparative magnitude of cross-strain conservation of HIV variable loop neutralization epitopes.PLoS One. 2010 Dec 29;5(12):e15994. doi: 10.1371/journal.pone.0015994. PLoS One. 2010. PMID: 21209919 Free PMC article.
-
HIV-specific CD4-induced Antibodies Mediate Broad and Potent Antibody-dependent Cellular Cytotoxicity Activity and Are Commonly Detected in Plasma From HIV-infected humans.EBioMedicine. 2015 Oct;2(10):1464-77. doi: 10.1016/j.ebiom.2015.09.001. EBioMedicine. 2015. PMID: 26629541 Free PMC article.
-
The presence of glutamine at position 315 but not epitope masking predominantly hinders HIV subtype C neutralization by the anti-V3 antibody B4e8.Virology. 2014 Aug;462-463:98-106. doi: 10.1016/j.virol.2014.05.034. Epub 2014 Jun 25. Virology. 2014. PMID: 24971702 Free PMC article.
-
Convergent Evolution of Neutralizing Antibodies to Staphylococcus aureus γ-Hemolysin C That Recognize an Immunodominant Primary Sequence-Dependent B-Cell Epitope.mBio. 2020 Jun 16;11(3):e00460-20. doi: 10.1128/mBio.00460-20. mBio. 2020. PMID: 32546616 Free PMC article.
References
-
- Barin F. Lahbabi Y. Buzelay L. Lejeune B. Baillou-Beaufils A. Denis F. Mathiot C. M'Boup S. Vithayasai V. Dietrich U. Goudeau A. Diversity of antibody binding to V3 peptides representing consensus sequences of HIV type 1 genotypes A to E: An approach for HIV type 1 serological subtyping. AIDS Res Hum Retroviruses. 1996;12(13):1279–1289. - PubMed
-
- Mascola JR. Louwagie J. McCutchan FE. Fischer CL. Hegerich PA. Wagner KF. Fowler AK. McNeil JG. Burke DS. Two antigenically distinct subtypes of human immunodeficiency virus type 1: Viral genotype predicts neutralization serotype. J Infect Dis. 1994;169(1):48–54. - PubMed
-
- Pantophlet R. Burton DR. GP120: Target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–769. - PubMed
-
- Gorny MK. Xu JY. Karwowska S. Buchbinder A. Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150(2):635–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

