Identification of intracellular carriers for the endocannabinoid anandamide
- PMID: 19307565
- PMCID: PMC2669397
- DOI: 10.1073/pnas.0901515106
Identification of intracellular carriers for the endocannabinoid anandamide
Abstract
The endocannabinoid anandamide (arachidonoyl ethanolamide, AEA) is an uncharged neuromodulatory lipid that, similar to many neurotransmitters, is inactivated through its cellular uptake and subsequent catabolism. AEA is hydrolyzed by fatty acid amide hydrolase (FAAH), an enzyme localized on the endoplasmic reticulum. In contrast to most neuromodulators, the hydrophilic cytosol poses a diffusional barrier for the efficient delivery of AEA to its site of catabolism. Therefore, AEA likely traverses the cytosol with the assistance of an intracellular carrier that increases its solubility and rate of diffusion. To study this process, AEA uptake and hydrolysis were examined in COS-7 cells expressing FAAH restricted to the endoplasmic reticulum, mitochondria, or the Golgi apparatus. AEA hydrolysis was detectable at the earliest measurable time point (3 seconds), suggesting that COS-7 cells, normally devoid of an endocannabinoid system, possess an efficient cytosolic trafficking mechanism for AEA. Three fatty acid binding proteins (FABPs) known to be expressed in brain were examined as possible intracellular AEA carriers. AEA uptake and hydrolysis were significantly potentiated in N18TG2 neuroblastoma cells after overexpression of FABP5 or FABP7, but not FABP3. Similar results were observed in COS-7 cells stably expressing FAAH. Consistent with the roles of FABP as AEA carriers, administration of the competitive FABP ligand oleic acid or the selective non-lipid FABP inhibitor BMS309403 attenuated AEA uptake and hydrolysis by approximately 50% in N18TG2 and COS-7 cells. Taken together, FABPs represent the first proteins known to transport AEA from the plasma membrane to FAAH for inactivation and may therefore be novel pharmacological targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
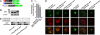
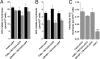
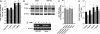
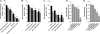
Similar articles
-
Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2.J Biol Chem. 2010 Jan 22;285(4):2796-806. doi: 10.1074/jbc.M109.058461. Epub 2009 Nov 19. J Biol Chem. 2010. PMID: 19926788 Free PMC article.
-
Inhibition of fatty acid binding proteins elevates brain anandamide levels and produces analgesia.PLoS One. 2014 Apr 4;9(4):e94200. doi: 10.1371/journal.pone.0094200. eCollection 2014. PLoS One. 2014. PMID: 24705380 Free PMC article.
-
Involvement of fatty acid amide hydrolase and fatty acid binding protein 5 in the uptake of anandamide by cell lines with different levels of fatty acid amide hydrolase expression: a pharmacological study.PLoS One. 2014 Jul 31;9(7):e103479. doi: 10.1371/journal.pone.0103479. eCollection 2014. PLoS One. 2014. PMID: 25078278 Free PMC article.
-
Anandamide uptake explained?Trends Pharmacol Sci. 2012 Apr;33(4):181-5. doi: 10.1016/j.tips.2012.01.001. Epub 2012 Jan 31. Trends Pharmacol Sci. 2012. PMID: 22297258 Review.
-
Organized trafficking of anandamide and related lipids.Vitam Horm. 2009;81:25-53. doi: 10.1016/S0083-6729(09)81002-9. Vitam Horm. 2009. PMID: 19647107 Review.
Cited by
-
Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones.Reprod Biol Endocrinol. 2011 Nov 29;9:152. doi: 10.1186/1477-7827-9-152. Reprod Biol Endocrinol. 2011. PMID: 22126420 Free PMC article.
-
Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery.Int J Mol Sci. 2022 Oct 30;23(21):13223. doi: 10.3390/ijms232113223. Int J Mol Sci. 2022. PMID: 36362014 Free PMC article. Review.
-
Inhibitors of monoacylglycerol lipase, fatty-acid amide hydrolase and endocannabinoid transport differentially suppress capsaicin-induced behavioral sensitization through peripheral endocannabinoid mechanisms.Pharmacol Res. 2010 Sep;62(3):249-58. doi: 10.1016/j.phrs.2010.03.007. Epub 2010 Apr 21. Pharmacol Res. 2010. PMID: 20416378 Free PMC article.
-
Involvement of TRPV1 Channels in Energy Homeostasis.Front Endocrinol (Lausanne). 2018 Jul 31;9:420. doi: 10.3389/fendo.2018.00420. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30108548 Free PMC article. Review.
-
Exploiting nanotechnologies and TRPV1 channels to investigate the putative anandamide membrane transporter.PLoS One. 2010 Apr 22;5(4):e10239. doi: 10.1371/journal.pone.0010239. PLoS One. 2010. PMID: 20422025 Free PMC article.
References
-
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. - PubMed
-
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. - PubMed
-
- de Lago E, Fernandez-Ruiz J, Ortega-Gutierrez S, Viso A, Lopez-Rodriguez ML, et al. UCM707, a potent and selective inhibitor of endocannabinoid uptake, potentiates hypokinetic and antinociceptive effects of anandamide. Eur J Pharmacol. 2002;449:99–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

