Zic1 levels regulate mossy fiber neuron position and axon laterality choice in the ventral brain stem
- PMID: 19303920
- PMCID: PMC2810558
- DOI: 10.1016/j.neuroscience.2009.02.082
Zic1 levels regulate mossy fiber neuron position and axon laterality choice in the ventral brain stem
Abstract
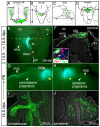
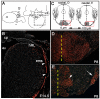
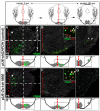
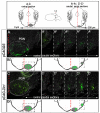
Pontine gray neurons of the brain stem are a major source of mossy fiber (MF) afferents to granule cells of the cerebellum. Achieving this connectivity involves an early regionalization of pontine gray neuron cell bodies within the brainstem pontine nuclei, as well as establishing the proper ratio of crossed versus uncrossed MF projections to contralateral versus ipsilateral cerebellar territories. Here, we report expression of the transcription factor Zic1 in newly postmitotic pontine gray neurons and present functional experiments in embryonic and postnatal mice that implicate Zic1 levels as a key determinant of pontine neuron cell body position within the pons and axon laterality. Reducing Zic1 levels embryonically via in utero electroporation of short hairpin RNA interference (shRNAi) vectors shifted the postnatal distribution of pontine neurons from caudolateral to rostromedial territories; by contrast, increasing Zic1 levels resulted in the reciprocal shift, with electroporated cells redistributing caudolaterally. Associated with the latter was a change in axon laterality, with a greater proportion of marked projections now targeting the ipsilateral instead of contralateral cerebellum. Zic1 levels in pontine gray neurons, therefore, play an important role in the development of pontocerebellar circuitry.
Figures

Similar articles
-
Cerebellar target neurons provide a stop signal for afferent neurite extension in vitro.J Neurosci. 1992 Feb;12(2):619-34. doi: 10.1523/JNEUROSCI.12-02-00619.1992. J Neurosci. 1992. PMID: 1740694 Free PMC article.
-
Collapsin-1/semaphorin-III/D is regulated developmentally in Purkinje cells and collapses pontocerebellar mossy fiber neuronal growth cones.J Neurosci. 1999 Jun 1;19(11):4437-48. doi: 10.1523/JNEUROSCI.19-11-04437.1999. J Neurosci. 1999. PMID: 10341245 Free PMC article.
-
The stop signal revised: immature cerebellar granule neurons in the external germinal layer arrest pontine mossy fiber growth.J Neurosci. 2006 May 31;26(22):6040-51. doi: 10.1523/JNEUROSCI.4815-05.2006. J Neurosci. 2006. PMID: 16738247 Free PMC article.
-
The Long Journey of Pontine Nuclei Neurons: From Rhombic Lip to Cortico-Ponto-Cerebellar Circuitry.Front Neural Circuits. 2017 May 17;11:33. doi: 10.3389/fncir.2017.00033. eCollection 2017. Front Neural Circuits. 2017. PMID: 28567005 Free PMC article. Review.
-
Organization of the pontine nuclei.Neurosci Res. 1992 Mar;13(2):83-118. doi: 10.1016/0168-0102(92)90092-q. Neurosci Res. 1992. PMID: 1374872 Review.
Cited by
-
Subtypes of medulloblastoma have distinct developmental origins.Nature. 2010 Dec 23;468(7327):1095-9. doi: 10.1038/nature09587. Epub 2010 Dec 8. Nature. 2010. PMID: 21150899 Free PMC article.
-
Conditional Loss of Hoxa5 Function Early after Birth Impacts on Expression of Genes with Synaptic Function.Front Mol Neurosci. 2017 Nov 15;10:369. doi: 10.3389/fnmol.2017.00369. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29187810 Free PMC article.
-
Rautenlippe Redux -- toward a unified view of the precerebellar rhombic lip.Curr Opin Cell Biol. 2009 Dec;21(6):741-7. doi: 10.1016/j.ceb.2009.10.003. Epub 2009 Oct 31. Curr Opin Cell Biol. 2009. PMID: 19883998 Free PMC article. Review.
-
Axonal patterns and targets of dA1 interneurons in the chick hindbrain.J Neurosci. 2012 Apr 25;32(17):5757-71. doi: 10.1523/JNEUROSCI.4231-11.2012. J Neurosci. 2012. PMID: 22539838 Free PMC article.
-
Axonal Projection Patterns of the Dorsal Interneuron Populations in the Embryonic Hindbrain.Front Neuroanat. 2021 Dec 24;15:793161. doi: 10.3389/fnana.2021.793161. eCollection 2021. Front Neuroanat. 2021. PMID: 35002640 Free PMC article. Review.
References
-
- Alcantara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127:1359–72. - PubMed
-
- Altman J, Bayer S. Development of the cerebellar system: in relation to its evolution, structure, and functions. CRC Press; Boca Raton, Florida: 1997.
-
- Altman J, Bayer SA. Development of the precerebellar nuclei in the rat: III. The posterior precerebellar extramural migratory stream and the lateral reticular and external cuneate nuclei. J Comp Neurol. 1987a;257:513–28. - PubMed
-
- Altman J, Bayer SA. Development of the precerebellar nuclei in the rat: IV. The anterior precerebellar extramural migratory stream and the nucleus reticularis tegmenti pontis and the basal pontine gray. J Comp Neurol. 1987b;257:529–52. - PubMed
-
- Aruga J. The role of Zic genes in neural development. Mol Cell Neurosci. 2004;26:205–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

