B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus
- PMID: 19299721
- PMCID: PMC3395367
- DOI: 10.4049/jimmunol.0800771
B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus
Abstract
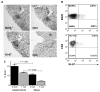
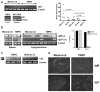
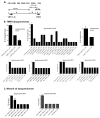
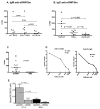
Intraperitoneal exposure of nonautoimmune mice to 2,6,10,14-tetramethylpentadecane (TMPD) causes lupus and the formation of ectopic lymphoid tissue. Although associated with humoral autoimmunity, it is not known whether Ab responses develop within ectopic lymphoid tissue or if B cells only secondarily migrate there. We show that ectopic lymphoid tissue induced by TMPD not only resembles secondary lymphoid tissue morphologically, but it also displays characteristics of germinal center reactions. Proliferating T and B lymphocytes were found within ectopic lymphoid tissue, activation-induced cytidine deaminase was expressed, and class-switched B cells were present. The presence of circular DNA intermediates, a hallmark of active class switch recombination, suggested that class switching occurs within the ectopic lymphoid tissue. Individual collections of ectopic lymphoid tissue ("lipogranulomas") from the same mouse contained different B cell repertoires, consistent with local germinal center-like reactions. Class-switched anti-RNP autoantibody-producing cells were also found in the lipogranulomas. Somatic hypermutation in the lipogranulomas was T cell-dependent, as was the production of isotype-switched anti-Sm/RNP autoantibodies. Thus, ectopic lymphoid tissue induced by TMPD recapitulates many of the functional characteristics of secondary lymphoid tissue and contains autoantibody-secreting cells, which may escape from normal censoring mechanisms in this location.
Figures


Similar articles
-
Maintenance of anti-Sm/RNP autoantibody production by plasma cells residing in ectopic lymphoid tissue and bone marrow memory B cells.J Immunol. 2013 Apr 15;190(8):3916-27. doi: 10.4049/jimmunol.1201880. Epub 2013 Mar 18. J Immunol. 2013. PMID: 23509349 Free PMC article.
-
Production of IgG autoantibody requires expression of activation-induced deaminase in early-developing B cells in a mouse model of SLE.Eur J Immunol. 2014 Oct;44(10):3093-108. doi: 10.1002/eji.201344282. Epub 2014 Aug 19. Eur J Immunol. 2014. PMID: 25044405 Free PMC article.
-
Colocalization of antigen-specific B and T cells within ectopic lymphoid tissue following immunization with exogenous antigen.J Immunol. 2008 Sep 1;181(5):3259-67. doi: 10.4049/jimmunol.181.5.3259. J Immunol. 2008. PMID: 18713997 Free PMC article.
-
B-cell selection and the development of autoantibodies.Arthritis Res Ther. 2012;14 Suppl 4(Suppl 4):S1. doi: 10.1186/ar3918. Epub 2012 Nov 2. Arthritis Res Ther. 2012. PMID: 23281837 Free PMC article. Review.
-
What do somatic hypermutation and class switch recombination teach us about chronic lymphocytic leukaemia pathogenesis?Curr Top Microbiol Immunol. 2005;294:71-89. doi: 10.1007/3-540-29933-5_5. Curr Top Microbiol Immunol. 2005. PMID: 16323428 Review.
Cited by
-
Excessive CD11c+Tbet+ B cells promote aberrant TFH differentiation and affinity-based germinal center selection in lupus.Proc Natl Acad Sci U S A. 2019 Sep 10;116(37):18550-18560. doi: 10.1073/pnas.1901340116. Epub 2019 Aug 26. Proc Natl Acad Sci U S A. 2019. PMID: 31451659 Free PMC article.
-
Mechanisms of tumor necrosis factor α antagonist-induced lupus in a murine model.Arthritis Rheumatol. 2015 Jan;67(1):225-37. doi: 10.1002/art.38882. Arthritis Rheumatol. 2015. PMID: 25252121 Free PMC article.
-
Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production.Eur J Immunol. 2016 Jan;46(1):81-91. doi: 10.1002/eji.201545673. Epub 2015 Nov 2. Eur J Immunol. 2016. PMID: 26474728 Free PMC article.
-
B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue.JCI Insight. 2016 Dec 8;1(20):e87234. doi: 10.1172/jci.insight.87234. JCI Insight. 2016. PMID: 27942581 Free PMC article.
-
Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity.Immunity. 2020 Dec 15;53(6):1136-1150. doi: 10.1016/j.immuni.2020.11.006. Immunity. 2020. PMID: 33326765 Free PMC article. Review.
References
-
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. - PubMed
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. - PubMed
-
- Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. - PubMed
-
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. - PubMed
Reference List
-
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

