Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets
- PMID: 19299711
- PMCID: PMC2659470
- DOI: 10.4049/jimmunol.0803982
Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets
Abstract
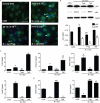
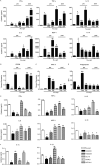
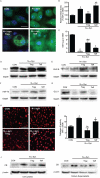
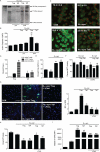
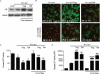
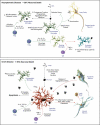
Microglial inflammatory neuroregulatory activities affect the tempo of nigrostriatal degeneration during Parkinson's disease (PD). Such activities are induced, in part, by misfolded, nitrated alpha-synuclein (N-alpha-syn) within Lewy bodies released from dying or dead dopaminergic neurons. Such pathobiological events initiate innate and adaptive immune responses affecting neurodegeneration. We posit that the neurobiological activities of activated microglia are affected by cell-protein and cell-cell contacts, in that microglial interactions with N-alpha-syn and CD4(+) T cells substantively alter the microglial proteome. This leads to alterations in cell homeostatic functions and disease. CD4(+)CD25(+) regulatory T cells suppress N-alpha-syn microglial-induced reactive oxygen species and NF-kappaB activation by modulating redox-active enzymes, cell migration, phagocytosis, and bioenergetic protein expression and cell function. In contrast, CD4(+)CD25(-) effector T cells exacerbate microglial inflammation and induce putative neurotoxic responses. These data support the importance of adaptive immunity in the regulation of Parkinson's disease-associated microglial inflammation.
Figures

Similar articles
-
Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells.J Proteome Res. 2009 Jul;8(7):3497-511. doi: 10.1021/pr9001614. J Proteome Res. 2009. PMID: 19432400 Free PMC article.
-
Nitrated alpha-synuclein-activated microglial profiling for Parkinson's disease.J Neurochem. 2008 Mar;104(6):1504-25. doi: 10.1111/j.1471-4159.2007.05087.x. Epub 2007 Nov 22. J Neurochem. 2008. PMID: 18036154
-
Nitrated alpha-synuclein and microglial neuroregulatory activities.J Neuroimmune Pharmacol. 2008 Jun;3(2):59-74. doi: 10.1007/s11481-008-9100-z. Epub 2008 Jan 17. J Neuroimmune Pharmacol. 2008. PMID: 18202920 Free PMC article.
-
Pathological α-synuclein exacerbates the progression of Parkinson's disease through microglial activation.Toxicol Lett. 2017 Jan 4;265:30-37. doi: 10.1016/j.toxlet.2016.11.002. Epub 2016 Nov 16. Toxicol Lett. 2017. PMID: 27865851 Review.
-
Differentiation and regulation of CD4+ T cell subsets in Parkinson's disease.Cell Mol Life Sci. 2024 Aug 17;81(1):352. doi: 10.1007/s00018-024-05402-0. Cell Mol Life Sci. 2024. PMID: 39153043 Free PMC article. Review.
Cited by
-
Biomarkers and the Role of α-Synuclein in Parkinson's Disease.Front Aging Neurosci. 2021 Mar 23;13:645996. doi: 10.3389/fnagi.2021.645996. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33833675 Free PMC article.
-
Taking the Perspective that a Depressive State Reflects Inflammation: Implications for the Use of Antidepressants.Front Psychol. 2012 Aug 17;3:297. doi: 10.3389/fpsyg.2012.00297. eCollection 2012. Front Psychol. 2012. PMID: 22912626 Free PMC article.
-
Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke.Immunity. 2021 Jul 13;54(7):1527-1542.e8. doi: 10.1016/j.immuni.2021.04.022. Epub 2021 May 19. Immunity. 2021. PMID: 34015256 Free PMC article.
-
Control of neuroinflammation as a therapeutic strategy for amyotrophic lateral sclerosis and other neurodegenerative disorders.Exp Neurol. 2010 Mar;222(1):1-5. doi: 10.1016/j.expneurol.2009.12.018. Epub 2010 Jan 4. Exp Neurol. 2010. PMID: 20044993 Free PMC article.
-
Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention.Neurobiol Dis. 2010 Mar;37(3):510-8. doi: 10.1016/j.nbd.2009.11.004. Epub 2009 Nov 10. Neurobiol Dis. 2010. PMID: 19913097 Free PMC article. Review.
References
-
- Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54:1916–1921. - PubMed
-
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. - PubMed
-
- Krishnan S, Chi EY, Wood SJ, Kendrick BS, Li C, Garzon-Rodriguez W, Wypych J, Randolph TW, Narhi LO, Biere AL, Citron M, Carpenter JF. Oxidative dimer formation is the critical rate-limiting step for Parkinson's disease alpha-synuclein fibrillogenesis. Biochemistry. 2003;42:829–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 NS043011-01/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P20 RR015635-04/RR/NCRR NIH HHS/United States
- 5P01NS31492/NS/NINDS NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- U54 NS043011/NS/NINDS NIH HHS/United States
- P01 NS043985-07/NS/NINDS NIH HHS/United States
- P01 MH064570-049001/MH/NIMH NIH HHS/United States
- R01 NS034239-14/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- U54NS43011/NS/NINDS NIH HHS/United States
- U54 NS043011-07S1/NS/NINDS NIH HHS/United States
- P01 NS043985-06A10006/NS/NINDS NIH HHS/United States
- P01MH64570/MH/NIMH NIH HHS/United States
- R37 NS036126-09/NS/NINDS NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- P20RR15635/RR/NCRR NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R37 NS036126-11/NS/NINDS NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
- 2R37 NS36126/NS/NINDS NIH HHS/United States
- P01 NS031492-150008/NS/NINDS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

