Regulation of the catalytic activity of herpes simplex virus 1 protein kinase Us3 by autophosphorylation and its role in pathogenesis
- PMID: 19297494
- PMCID: PMC2681960
- DOI: 10.1128/JVI.00103-09
Regulation of the catalytic activity of herpes simplex virus 1 protein kinase Us3 by autophosphorylation and its role in pathogenesis
Abstract
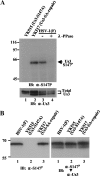
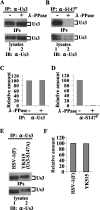
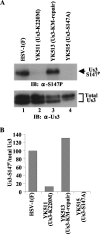
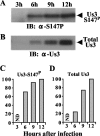
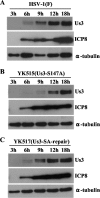
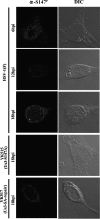
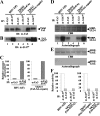
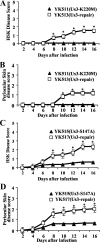
Us3 is a serine/threonine protein kinase encoded by herpes simplex virus 1 (HSV-1). We recently identified serine at Us3 position 147 (Ser-147) as a physiological phosphorylation site of Us3 (A. Kato, M. Tanaka, M. Yamamoto, R. Asai, T. Sata, Y. Nishiyama, and Y. Kawaguchi, J. Virol. 82:6172-6189, 2008). In the present study, we investigated the effects of phosphorylation of Us3 Ser-147 on regulation of Us3 catalytic activity in infected cells and on HSV-1 pathogenesis. Our results were as follows. (i) Only a small fraction of Us3 purified from infected cells was phosphorylated at Ser-147. (ii) Us3 phosphorylated at Ser-147 purified from infected cells had significantly higher kinase activity than Us3 not phosphorylated at Ser-147. (iii) Phosphorylation of Us3 Ser-147 in infected cells was dependent on Us3 kinase activity. (iv) Replacement of Us3 Ser-147 by alanine significantly reduced viral replication in the mouse cornea and the development of herpes stromal keratitis and periocular skin disease in mice. These results indicated that Us3 catalytic activity is tightly regulated by autophosphorylation of Ser-147 in infected cells and that regulation of Us3 activity by autophosphorylation appeared to play a critical role in viral replication in vivo and in HSV-1 pathogenesis.
Figures

Similar articles
-
Effects of phosphorylation of herpes simplex virus 1 envelope glycoprotein B by Us3 kinase in vivo and in vitro.J Virol. 2010 Jan;84(1):153-62. doi: 10.1128/JVI.01447-09. J Virol. 2010. PMID: 19846518 Free PMC article.
-
Roles of Us8A and Its Phosphorylation Mediated by Us3 in Herpes Simplex Virus 1 Pathogenesis.J Virol. 2016 May 27;90(12):5622-5635. doi: 10.1128/JVI.00446-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27030266 Free PMC article.
-
Phosphorylation of a herpes simplex virus 1 dUTPase by a viral protein kinase, Us3, dictates viral pathogenicity in the central nervous system but not at the periphery.J Virol. 2014 Mar;88(5):2775-85. doi: 10.1128/JVI.03300-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352467 Free PMC article.
-
Us3 Protein Kinase Encoded by HSV: The Precise Function and Mechanism on Viral Life Cycle.Adv Exp Med Biol. 2018;1045:45-62. doi: 10.1007/978-981-10-7230-7_3. Adv Exp Med Biol. 2018. PMID: 29896662 Review.
-
[Molecular mechanism by which Us3 protein kinase regulates the pathogenicity of herpes simplex virus type-1].Uirusu. 2016;66(1):83-90. doi: 10.2222/jsv.66.83. Uirusu. 2016. PMID: 28484184 Review. Japanese.
Cited by
-
Effects of phosphorylation of herpes simplex virus 1 envelope glycoprotein B by Us3 kinase in vivo and in vitro.J Virol. 2010 Jan;84(1):153-62. doi: 10.1128/JVI.01447-09. J Virol. 2010. PMID: 19846518 Free PMC article.
-
The alphaherpesvirus US3/ORF66 protein kinases direct phosphorylation of the nuclear matrix protein matrin 3.J Virol. 2011 Jan;85(1):568-81. doi: 10.1128/JVI.01611-10. Epub 2010 Oct 20. J Virol. 2011. PMID: 20962082 Free PMC article.
-
pUL21 is a viral phosphatase adaptor that promotes herpes simplex virus replication and spread.PLoS Pathog. 2021 Aug 16;17(8):e1009824. doi: 10.1371/journal.ppat.1009824. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34398933 Free PMC article.
-
VHS, US3 and UL13 viral tegument proteins are required for Herpes Simplex Virus-Induced modification of protein kinase R.Sci Rep. 2020 Mar 27;10(1):5580. doi: 10.1038/s41598-020-62619-2. Sci Rep. 2020. PMID: 32221365 Free PMC article.
-
A single-amino-acid substitution in herpes simplex virus 1 envelope glycoprotein B at a site required for binding to the paired immunoglobulin-like type 2 receptor alpha (PILRalpha) abrogates PILRalpha-dependent viral entry and reduces pathogenesis.J Virol. 2010 Oct;84(20):10773-83. doi: 10.1128/JVI.01166-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686018 Free PMC article.
References
-
- Daikoku, T., Y. Yamashita, T. Tsurumi, K. Maeno, and Y. Nishiyama. 1993. Purification and biochemical characterization of the protein kinase encoded by the US3 gene of herpes simplex virus type 2. Virology 197685-694. - PubMed
-
- Edelman, A. M., D. K. Blumenthal, and E. G. Krebs. 1987. Protein serine/threonine kinases. Annu. Rev. Biochem. 56567-613. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

