Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1
- PMID: 19293155
- PMCID: PMC2682873
- DOI: 10.1074/jbc.M806902200
Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1
Abstract
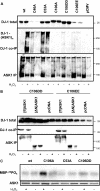
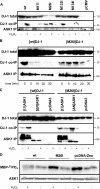
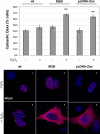
Parkinson disease (PD)-associated genomic deletions and the destabilizing L166P point mutation lead to loss of the cytoprotective DJ-1 protein. The effects of other PD-associated point mutations are less clear. Here we demonstrate that the M26I mutation reduces DJ-1 expression, particularly in a null background (knockout mouse embryonic fibroblasts). Thus, homozygous M26I mutation causes loss of DJ-1 protein. To determine the cellular consequences, we measured suppression of apoptosis signal-regulating kinase 1 (ASK1) and cytotoxicity for [M26I]DJ-1, and systematically all other DJ-1 methionine and cysteine mutants. C106A mutation of the central redox site specifically abolished binding to ASK1 and the cytoprotective activity of DJ-1. DJ-1 was apparently recruited into the ASK1 signalosome via Cys-106-linked mixed disulfides. The designed higher order oxidation mimicking [C106DD]DJ-1 non-covalently bound to ASK1 even in the absence of hydrogen peroxide and conferred partial cytoprotection. Interestingly, mutations of peripheral redox sites (C46A and C53A) and M26I also led to constitutive ASK1 binding. Cytoprotective [wt]DJ-1 bound to the ASK1 N terminus (which is known to bind another negative regulator, thioredoxin 1), whereas [M26I]DJ-1 bound to aberrant C-terminal site(s). Consequently, the peripheral cysteine mutants retained cytoprotective activity, whereas the PD-associated mutant [M26I]DJ-1 failed to suppress ASK1 activity and nuclear export of the death domain-associated protein Daxx and did not promote cytoprotection. Thus, cytoprotective binding of DJ-1 to ASK1 depends on the central redox-sensitive Cys-106 and may be modulated by peripheral cysteine residues. We suggest that impairments in oxidative conformation changes of DJ-1 might contribute to PD neurodegeneration.
Figures


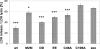
Similar articles
-
DJ-1 protects against oxidative damage by regulating the thioredoxin/ASK1 complex.Neurosci Res. 2010 Jul;67(3):203-8. doi: 10.1016/j.neures.2010.04.002. Epub 2010 Apr 10. Neurosci Res. 2010. PMID: 20385180 Free PMC article.
-
Structural determinants of the C-terminal helix-kink-helix motif essential for protein stability and survival promoting activity of DJ-1.J Biol Chem. 2007 May 4;282(18):13680-91. doi: 10.1074/jbc.M609821200. Epub 2007 Mar 1. J Biol Chem. 2007. PMID: 17331951
-
Redox activated MAP kinase death signaling cascade initiated by ASK1 is not activated in female mice following MPTP: novel mechanism of neuroprotection.Neurotox Res. 2009 Aug;16(2):116-26. doi: 10.1007/s12640-009-9058-5. Epub 2009 May 1. Neurotox Res. 2009. PMID: 19526288
-
Cytoprotective mechanisms of DJ-1 against oxidative stress through modulating ERK1/2 and ASK1 signal transduction.Redox Biol. 2018 Apr;14:211-217. doi: 10.1016/j.redox.2017.09.008. Epub 2017 Sep 18. Redox Biol. 2018. PMID: 28954246 Free PMC article. Review.
-
The role of cysteine oxidation in DJ-1 function and dysfunction.Antioxid Redox Signal. 2011 Jul 1;15(1):111-22. doi: 10.1089/ars.2010.3481. Epub 2011 Jan 14. Antioxid Redox Signal. 2011. PMID: 20812780 Free PMC article. Review.
Cited by
-
The Role of Oxidative Stress in Parkinson's Disease.Antioxidants (Basel). 2020 Jul 8;9(7):597. doi: 10.3390/antiox9070597. Antioxidants (Basel). 2020. PMID: 32650609 Free PMC article. Review.
-
DJ-1 is not a deglycase and makes a modest contribution to cellular defense against methylglyoxal damage in neurons.J Neurochem. 2022 Aug;162(3):245-261. doi: 10.1111/jnc.15656. Epub 2022 Jul 2. J Neurochem. 2022. PMID: 35713360 Free PMC article.
-
Stepwise oxidations play key roles in the structural and functional regulations of DJ-1.Biochem J. 2021 Oct 15;478(19):3505-3525. doi: 10.1042/BCJ20210245. Biochem J. 2021. PMID: 34515295 Free PMC article.
-
Primary skin fibroblasts as a model of Parkinson's disease.Mol Neurobiol. 2012 Aug;46(1):20-7. doi: 10.1007/s12035-012-8245-1. Epub 2012 Feb 19. Mol Neurobiol. 2012. PMID: 22350618 Free PMC article. Review.
-
Drosophila DJ-1 decreases neural sensitivity to stress by negatively regulating Daxx-like protein through dFOXO.PLoS Genet. 2013 Apr;9(4):e1003412. doi: 10.1371/journal.pgen.1003412. Epub 2013 Apr 4. PLoS Genet. 2013. PMID: 23593018 Free PMC article.
References
-
- Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O., Breedveld, G. J., Krieger, E., Dekker, M. C. J., Squitieri, F., Ibanez, P., Joosse, M., van Dongen, J. W., Vanacore, N., van Swieten, J. C., Brice, A., Meco, G., van Duijn, C. M., Oostra, B. A., and Heutink, P. (2003) Science 299 256–259 - PubMed
-
- Macedo, M. G., Anar, B., Bronner, I. F., Cannella, M., Squitieri, F., Bonifati, V., Hoogeveen, A., Heutink, P., and Rizzu, P. (2003) Hum. Mol. Genet. 12 2807–2816 - PubMed
-
- Miller, D. W., Ahmad, R., Hague, S., Baptista, M. J., Canet-Avilés, R., McLendon, C., Carter, D. M., Zhu, P.-P., Stadler, J., Chandran, J., Klinefelter, G. R., Blackstone, C., and Cookson, M. R. (2003) J. Biol. Chem. 278 36588–36595 - PubMed
-
- Moore, D. J., Zhang, L., Dawson, T. M., and Dawson, V. L. (2003) J. Neurochem. 87 1558–1567 - PubMed
-
- Olzmann, J. A., Brown, K., Wilkinson, K. D., Rees, H. D., Huai, Q., Ke, H., Levey, A. I., Li, L., and Chin, L.-S. (2004) J. Biol. Chem. 279 8506–8515 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

