A role for CD81 on the late steps of HIV-1 replication in a chronically infected T cell line
- PMID: 19284574
- PMCID: PMC2657109
- DOI: 10.1186/1742-4690-6-28
A role for CD81 on the late steps of HIV-1 replication in a chronically infected T cell line
Abstract
Background: HIV-1 uses cellular co-factors for virion formation and release. The virus is able to incorporate into the viral particles host cellular proteins, such as tetraspanins which could serve to facilitate HIV-1 egress. Here, we investigated the implication of several tetraspanins on HIV-1 formation and release in chronically infected T-lymphoblastic cells, a model that permits the study of the late steps of HIV-1 replication.
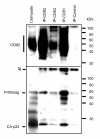
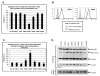
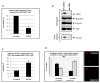
Results: Our data revealed that HIV-1 Gag and Env structural proteins co-localized with tetraspanins in the form of clusters. Co-immunoprecipitation experiments showed that Gag proteins interact, directly or indirectly, with CD81, and less with CD82, in tetraspanin-enriched microdomains composed of CD81/CD82/CD63. In addition, when HIV-1 producing cells were treated with anti-CD81 antibodies, or upon CD81 silencing by RNA interference, HIV-1 release was significantly impaired, and its infectivity was modulated. Finally, CD81 downregulation resulted in Gag redistribution at the cell surface.
Conclusion: Our findings not only extend the notion that HIV-1 assembly can occur on tetraspanin-enriched microdomains in T cells, but also highlight a critical role for the tetraspanin CD81 on the late steps of HIV replication.
Figures
Similar articles
-
Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains.J Virol. 2007 Aug;81(15):7873-84. doi: 10.1128/JVI.01845-06. Epub 2007 May 23. J Virol. 2007. PMID: 17522207 Free PMC article.
-
Human Discs Large is a new negative regulator of human immunodeficiency virus-1 infectivity.Mol Biol Cell. 2009 Jan;20(1):498-508. doi: 10.1091/mbc.e08-02-0189. Epub 2008 Oct 22. Mol Biol Cell. 2009. PMID: 18946087 Free PMC article.
-
Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins.J Virol. 2008 Jan;82(2):1021-33. doi: 10.1128/JVI.01044-07. Epub 2007 Nov 7. J Virol. 2008. PMID: 17989173 Free PMC article.
-
Tetraspanin functions during HIV-1 and influenza virus replication.Biochem Soc Trans. 2011 Apr;39(2):529-31. doi: 10.1042/BST0390529. Biochem Soc Trans. 2011. PMID: 21428933 Free PMC article. Review.
-
Tetraspanins, Another Piece in the HIV-1 Replication Puzzle.Front Immunol. 2018 Aug 3;9:1811. doi: 10.3389/fimmu.2018.01811. eCollection 2018. Front Immunol. 2018. PMID: 30127789 Free PMC article. Review.
Cited by
-
Vpu is the main determinant for tetraspanin downregulation in HIV-1-infected cells.J Virol. 2015 Mar;89(6):3247-55. doi: 10.1128/JVI.03719-14. Epub 2015 Jan 7. J Virol. 2015. PMID: 25568205 Free PMC article.
-
Dual function of CD81 in influenza virus uncoating and budding.PLoS Pathog. 2013;9(10):e1003701. doi: 10.1371/journal.ppat.1003701. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24130495 Free PMC article.
-
Tetraspanins: Host Factors in Viral Infections.Int J Mol Sci. 2021 Oct 27;22(21):11609. doi: 10.3390/ijms222111609. Int J Mol Sci. 2021. PMID: 34769038 Free PMC article. Review.
-
Multiple Inhibitory Factors Act in the Late Phase of HIV-1 Replication: a Systematic Review of the Literature.Microbiol Mol Biol Rev. 2018 Jan 10;82(1):e00051-17. doi: 10.1128/MMBR.00051-17. Print 2018 Mar. Microbiol Mol Biol Rev. 2018. PMID: 29321222 Free PMC article. Review.
-
Tetraspanins regulate cell-to-cell transmission of HIV-1.Retrovirology. 2009 Jul 14;6:64. doi: 10.1186/1742-4690-6-64. Retrovirology. 2009. PMID: 19602278 Free PMC article.
References
-
- Delaguillaumie A, Harriague J, Kohanna S, Bismuth G, Rubinstein E, Seigneuret M, Conjeaud H. Tetraspanin CD82 controls the association of cholesterol-dependent microdomains with the actin cytoskeleton in T lymphocytes: relevance to co-stimulation. J Cell Sci. 2004;117:5269–5282. doi: 10.1242/jcs.01380. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

