Distinct functional and anatomical architecture of the endocannabinoid system in the auditory brainstem
- PMID: 19279154
- PMCID: PMC2681419
- DOI: 10.1152/jn.00047.2009
Distinct functional and anatomical architecture of the endocannabinoid system in the auditory brainstem
Erratum in
- J Neurophysiol. 2010 May;103(5):2931
Abstract
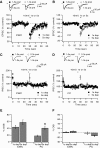
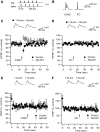
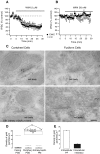
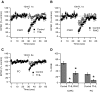
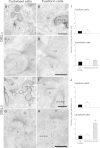
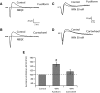
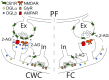
Endocannabinoids (ECs) act as retrograde messengers that enable postsynaptic cells to regulate the strength of their synaptic inputs. Here, by using physiological and histological techniques, we showed that, unlike in other parts of the brain, excitatory inputs are more sensitive than inhibitory inputs to EC signaling in the dorsal cochlear nucleus (DCN), an auditory brainstem nucleus. The principal cells of the DCN, fusiform cells, integrate acoustic signals through nonplastic synapses located in the deep layer with multimodal sensory signals carried by plastic parallel fibers in the molecular layer. Parallel fibers contact fusiform cells and inhibitory interneurons, the cartwheel cells, which in turn inhibit fusiform cells. Postsynaptic depolarization or pairing of postsynaptic potentials (PSPs) with action potentials (APs) induced EC-mediated modulation of excitatory inputs but did not affect inhibitory inputs. Quantitative electron microscopical studies showed that glutamatergic terminals express more cannabinoid 1 receptors (CB1Rs) than glycinergic terminals. Fusiform and cartwheel cells express diacylglycerol lipase alpha and beta (DGLalpha/beta), the two enzymes involved in the generation of the EC, 2-arachidonoyl-glycerol (2-AG). DGLalpha and DGLbeta are found in the spines of cartwheel but not fusiform cells indicating that the synthesis of ECs is more distant from parallel fiber synapses in fusiform than cartwheel cells. The differential localization and density of DGLalpha/beta and CB1Rs leads to cell- and input-specific EC signaling that favors activity-dependent EC-mediated suppression at synapses between parallel fibers and cartwheel cell spines, thus leading to reduced feedforward inhibition in fusiform cells. We propose that EC signaling is a major modulator of the balance of excitation and inhibition in auditory circuits.
Figures
Similar articles
-
Sustained firing of cartwheel cells in the dorsal cochlear nucleus evokes endocannabinoid release and retrograde suppression of parallel fiber synapses.J Neurosci. 2011 Nov 2;31(44):15807-17. doi: 10.1523/JNEUROSCI.4088-11.2011. J Neurosci. 2011. PMID: 22049424 Free PMC article.
-
Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses.Nat Neurosci. 2010 May;13(5):592-600. doi: 10.1038/nn.2517. Epub 2010 Mar 28. Nat Neurosci. 2010. PMID: 20348918 Free PMC article.
-
Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus.Nat Neurosci. 2004 Jul;7(7):719-25. doi: 10.1038/nn1272. Epub 2004 Jun 20. Nat Neurosci. 2004. PMID: 15208632
-
Context-dependent synaptic action of glycinergic and GABAergic inputs in the dorsal cochlear nucleus.J Neurosci. 1996 Apr 1;16(7):2208-19. doi: 10.1523/JNEUROSCI.16-07-02208.1996. J Neurosci. 1996. PMID: 8601801 Free PMC article.
-
Retrograde endocannabinoid signaling in the cerebellar cortex.Cerebellum. 2006;5(2):134-45. doi: 10.1080/14734220600791477. Cerebellum. 2006. PMID: 16818388 Review.
Cited by
-
Does cannabis alleviate tinnitus? A review of the current literature.Laryngoscope Investig Otolaryngol. 2020 Oct 30;5(6):1147-1155. doi: 10.1002/lio2.479. eCollection 2020 Dec. Laryngoscope Investig Otolaryngol. 2020. PMID: 33364406 Free PMC article. Review.
-
Sustained firing of cartwheel cells in the dorsal cochlear nucleus evokes endocannabinoid release and retrograde suppression of parallel fiber synapses.J Neurosci. 2011 Nov 2;31(44):15807-17. doi: 10.1523/JNEUROSCI.4088-11.2011. J Neurosci. 2011. PMID: 22049424 Free PMC article.
-
The localization and physiological effects of cannabinoid receptor 1 in the brain stem auditory system of the chick.Neuroscience. 2011 Oct 27;194:150-9. doi: 10.1016/j.neuroscience.2011.05.061. Epub 2011 Jun 7. Neuroscience. 2011. PMID: 21703331 Free PMC article.
-
Targeting the Limbic System: Insights into Its Involvement in Tinnitus.Int J Mol Sci. 2023 Jun 8;24(12):9889. doi: 10.3390/ijms24129889. Int J Mol Sci. 2023. PMID: 37373034 Free PMC article. Review.
-
Spontaneous spiking and synaptic depression underlie noradrenergic control of feed-forward inhibition.Neuron. 2011 Jul 28;71(2):306-18. doi: 10.1016/j.neuron.2011.05.039. Neuron. 2011. PMID: 21791289 Free PMC article.
References
-
- BellCCBell CC. Evolution of cerebellum-like structures. Brain Beh Evol 59: 312–326, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

