FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation
- PMID: 19276113
- PMCID: PMC2654023
- DOI: 10.1073/pnas.0901104106
FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation
Abstract
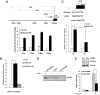
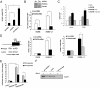
Loss-of-function mutations of phosphatase/tensin homolog deleted on chromosome 10 (PTEN)-induced putative kinase 1 (Pink1) (also known as Park6) identified in familial forms of Parkinson's disease (PD) are associated with compromised mitochondrial function. Emerging data suggest that Pink1 is an essential pro-survival factor that is induced in response to oxidative stress. However, the mechanisms regulating Pink1 expression under stress conditions remain unknown. Forkhead box, subgroup O (FOXO) transcription factors carry out distinct biological functions in response to different extracellular signals. Notably, FOXO factors possess evolutionarily conserved roles in protecting cells from oxidative stress-induced death. Here we report that the FOXO family member FOXO3a controls Pink1 transcription in both mouse and human cells subjected to growth factor deprivation and that this regulation is exerted through evolutionarily conserved FOXO binding elements. Induction of Pink1 by FOXO3a is crucial for survival signals in lymphocytes, as depletion of Pink1 sensitizes these cells to death induced by deprivation of an essential growth factor. Our data reveal that the role of FOXO factors in protecting cells from growth factor deprivation-triggered apoptosis has been underestimated and that FOXOs mediate this protection by transactivating anti-apoptotic effectors like Pink1. Given the essential role of Pink1 in combating cell death, our findings may help to dissect the mechanisms by which FOXO proteins function as anti-oxidative stress factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
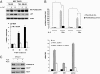

Similar articles
-
DJ1 represses glycolysis and cell proliferation by transcriptionally up-regulating Pink1.Biochem J. 2015 Apr 15;467(2):303-10. doi: 10.1042/BJ20141025. Biochem J. 2015. PMID: 25670069 Free PMC article.
-
Induction of Mxi1-SR alpha by FOXO3a contributes to repression of Myc-dependent gene expression.Mol Cell Biol. 2007 Jul;27(13):4917-30. doi: 10.1128/MCB.01789-06. Epub 2007 Apr 23. Mol Cell Biol. 2007. PMID: 17452451 Free PMC article.
-
PARK6 PINK1 mutants are defective in maintaining mitochondrial membrane potential and inhibiting ROS formation of substantia nigra dopaminergic neurons.Biochim Biophys Acta. 2011 Jun;1812(6):674-84. doi: 10.1016/j.bbadis.2011.03.007. Epub 2011 Mar 21. Biochim Biophys Acta. 2011. PMID: 21421046
-
FOXO factors: a matter of life and death.Future Oncol. 2006 Feb;2(1):83-9. doi: 10.2217/14796694.2.1.83. Future Oncol. 2006. PMID: 16556075 Review.
-
A new fork for clinical application: targeting forkhead transcription factors in cancer.Clin Cancer Res. 2009 Feb 1;15(3):752-7. doi: 10.1158/1078-0432.CCR-08-0124. Clin Cancer Res. 2009. PMID: 19188143 Free PMC article. Review.
Cited by
-
Programmed cell death in Parkinson's disease.Cold Spring Harb Perspect Med. 2012 Aug 1;2(8):a009365. doi: 10.1101/cshperspect.a009365. Cold Spring Harb Perspect Med. 2012. PMID: 22908196 Free PMC article. Review.
-
Pharmacological, Biochemical and Immunological Studies on Protective Effect of Mangiferin in 6-Hydroxydopamine (6-OHDA)-Induced Parkinson's Disease in Rats.Ann Neurosci. 2021 Jul;28(3-4):137-149. doi: 10.1177/09727531211051976. Epub 2021 Nov 2. Ann Neurosci. 2021. PMID: 35341236 Free PMC article.
-
Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney.J Clin Invest. 2010 Apr;120(4):1043-55. doi: 10.1172/JCI41376. Epub 2010 Mar 24. J Clin Invest. 2010. PMID: 20335657 Free PMC article.
-
Mitochondrial impairment increases FL-PINK1 levels by calcium-dependent gene expression.Neurobiol Dis. 2014 Feb;62:426-40. doi: 10.1016/j.nbd.2013.10.021. Epub 2013 Oct 29. Neurobiol Dis. 2014. PMID: 24184327 Free PMC article.
-
Decoding Common Features of Neurodegenerative Disorders: From Differentially Expressed Genes to Pathways.Curr Genomics. 2018 May;19(4):300-312. doi: 10.2174/1389202918666171005100549. Curr Genomics. 2018. PMID: 29755292 Free PMC article.
References
-
- Unoki M, Nakamura Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene. 2001;20(33):4457–4465. - PubMed
-
- Petit A, et al. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280(40):34025–34032. - PubMed
-
- MacKeigan JP, Murphy LO, Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol. 2005;7(6):591–600. - PubMed
-
- Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 2005;337(4):1133–1138. - PubMed
-
- Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

