Huntingtin as an essential integrator of intracellular vesicular trafficking
- PMID: 19269181
- PMCID: PMC2930405
- DOI: 10.1016/j.tcb.2009.01.005
Huntingtin as an essential integrator of intracellular vesicular trafficking
Abstract
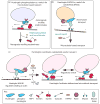
The neurodegenerative disorder Huntington's disease is caused by an expansion in the polyglutamine repeat region of the protein huntingtin. Multiple studies in cellular and animal model systems indicate that this mutation imparts a novel toxic function required for disease pathogenesis. However, the normal function of huntingtin, an essential cellular protein in higher vertebrates, is not yet well understood. Emerging data indicate an important role for wild-type huntingtin in the intracellular transport of vesicles and organelles. Here, we discuss current progress on the role of huntingtin in vesicular trafficking, focusing on the proposal that huntingtin might be a crucial regulator of organelle transport along the cellular cytoskeleton.
Figures
Similar articles
-
Huntingtin: an iron-regulated protein essential for normal nuclear and perinuclear organelles.Hum Mol Genet. 2000 Nov 22;9(19):2789-97. doi: 10.1093/hmg/9.19.2789. Hum Mol Genet. 2000. PMID: 11092755
-
Hypothesis: Huntingtin may function in membrane association and vesicular trafficking.Biochem Cell Biol. 2006 Dec;84(6):912-7. doi: 10.1139/o06-181. Biochem Cell Biol. 2006. PMID: 17215878 Review.
-
Nucleocytoplasmic trafficking and transcription effects of huntingtin in Huntington's disease.Prog Neurobiol. 2007 Nov;83(4):211-27. doi: 10.1016/j.pneurobio.2006.11.004. Epub 2007 Jan 22. Prog Neurobiol. 2007. PMID: 17240517 Review.
-
Mutant huntingtin interacts with {beta}-tubulin and disrupts vesicular transport and insulin secretion.Hum Mol Genet. 2009 Oct 15;18(20):3942-54. doi: 10.1093/hmg/ddp336. Epub 2009 Jul 23. Hum Mol Genet. 2009. PMID: 19628478
-
Huntingtin-protein interactions and the pathogenesis of Huntington's disease.Trends Genet. 2004 Mar;20(3):146-54. doi: 10.1016/j.tig.2004.01.008. Trends Genet. 2004. PMID: 15036808 Review.
Cited by
-
Retrograde NGF axonal transport--motor coordination in the unidirectional motility regime.Biophys J. 2015 Jun 2;108(11):2691-703. doi: 10.1016/j.bpj.2015.04.036. Biophys J. 2015. PMID: 26039170 Free PMC article.
-
Functional consequences of necdin nucleocytoplasmic localization.PLoS One. 2012;7(3):e33786. doi: 10.1371/journal.pone.0033786. Epub 2012 Mar 19. PLoS One. 2012. PMID: 22442722 Free PMC article.
-
Retrograde axonal transport: pathways to cell death?Trends Neurosci. 2010 Jul;33(7):335-44. doi: 10.1016/j.tins.2010.03.006. Epub 2010 Apr 29. Trends Neurosci. 2010. PMID: 20434225 Free PMC article. Review.
-
Changes in BiP availability reveal hypersensitivity to acute endoplasmic reticulum stress in cells expressing mutant huntingtin.J Cell Sci. 2011 Oct 1;124(Pt 19):3332-43. doi: 10.1242/jcs.087510. Epub 2011 Sep 6. J Cell Sci. 2011. PMID: 21896647 Free PMC article.
-
Cooperation of cell adhesion and autophagy in the brain: Functional roles in development and neurodegenerative disease.Matrix Biol Plus. 2021 Oct 23;12:100089. doi: 10.1016/j.mbplus.2021.100089. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34786551 Free PMC article. Review.
References
-
- Imarisio S, et al. Huntington’s disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. - PubMed
-
- Truant R, et al. Nucleocytoplasmic trafficking and transcription effects of huntingtin in Huntington’s disease. Prog Neurobiol. 2007;83:211–227. - PubMed
-
- DiFiglia M, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14:1075–1081. - PubMed
-
- Hoffner G, et al. Perinuclear localization of huntingtin as a consequence of its binding to microtubules through an interaction with beta-tubulin: relevance to Huntington’s disease. J Cell Sci. 2002;115:941–948. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

