Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms
- PMID: 19261878
- PMCID: PMC2673506
- DOI: 10.1523/JNEUROSCI.6186-08.2009
Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms
Abstract
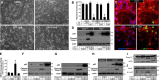
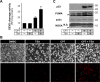
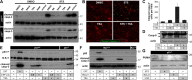
Pharmacological manipulation of protein acetylation levels by histone deacetylase (HDAC) inhibitors represents a novel therapeutic strategy to treat neurodegeneration as well as cancer. However, the molecular mechanisms that determine how HDAC inhibition exerts a protective effect in neurons as opposed to a cytotoxic action in tumor cells has not been elucidated. We addressed this issue in cultured postnatal mouse cortical neurons whose p53-dependent and p53-independent intrinsic apoptotic programs require the proapoptotic multidomain protein, Bax. Despite promoting nuclear p53 accumulation, Class I/II HDAC inhibitors (HDACIs) protected neurons from p53-dependent cell death induced by camptothecin, etoposide, heterologous p53 expression or the MDM2 inhibitor, nutlin-3a. HDACIs suppressed p53-dependent PUMA expression, a critical signaling intermediate linking p53 to Bax activation, thus preventing postmitochondrial events including cleavage of caspase-9 and caspase-3. In human SH-SY5Y neuroblastoma cells, however, HDACIs were not able to prevent p53-dependent cell death. Moreover, HDACIs also prevented caspase-3 cleavage in postnatal cortical neurons treated with staurosporine, 3-nitropropionic acid and a Bcl-2 inhibitor, all of which require the presence of Bax but not p53 to promote apoptosis. Although these three toxic agents displayed a requirement for Bax, they did not promote PUMA induction. These results demonstrate that HDACIs block Bax-dependent cell death by two distinct mechanisms to prevent neuronal apoptosis, thus identifying for the first time a defined molecular target for their neuroprotective actions.
Figures
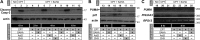
Similar articles
-
6-Hydroxydopamine activates the mitochondrial apoptosis pathway through p38 MAPK-mediated, p53-independent activation of Bax and PUMA.J Neurochem. 2008 Mar;104(6):1599-612. doi: 10.1111/j.1471-4159.2007.05115.x. Epub 2007 Nov 6. J Neurochem. 2008. PMID: 17996028
-
Inhibitors of histone deacetylase (HDAC) restore the p53 pathway in neuroblastoma cells.Br J Pharmacol. 2008 Feb;153(4):657-68. doi: 10.1038/sj.bjp.0707608. Epub 2007 Dec 3. Br J Pharmacol. 2008. PMID: 18059320 Free PMC article.
-
The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons.J Neurochem. 2006 Mar;96(5):1213-26. doi: 10.1111/j.1471-4159.2005.03676.x. J Neurochem. 2006. PMID: 16478523
-
Induction of PIG3 and NOXA through acetylation of p53 at 320 and 373 lysine residues as a mechanism for apoptotic cell death by histone deacetylase inhibitors.Cancer Res. 2003 Dec 15;63(24):8948-54. Cancer Res. 2003. PMID: 14695212
-
Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights.Pharmacol Ther. 2014 Sep;143(3):323-36. doi: 10.1016/j.pharmthera.2014.04.004. Epub 2014 Apr 24. Pharmacol Ther. 2014. PMID: 24769080 Free PMC article. Review.
Cited by
-
Dysregulation of histone deacetylases in carcinogenesis and tumor progression: a possible link to apoptosis and autophagy.Cell Mol Life Sci. 2019 Sep;76(17):3263-3282. doi: 10.1007/s00018-019-03098-1. Epub 2019 Apr 13. Cell Mol Life Sci. 2019. PMID: 30982077 Free PMC article. Review.
-
Effects of caffeine on cell viability and activity of histone deacetylase 1 and histone acetyltransferase in glioma cells.Tzu Chi Med J. 2016 Jul-Sep;28(3):103-108. doi: 10.1016/j.tcmj.2016.06.005. Epub 2016 Aug 1. Tzu Chi Med J. 2016. PMID: 28757735 Free PMC article.
-
Defining the role of histone deacetylases in the inhibition of mammary carcinogenesis by dietary energy restriction (DER): effects of suberoylanilide hydroxamic acid (SAHA) and DER in a rat model.Cancer Prev Res (Phila). 2013 Apr;6(4):290-8. doi: 10.1158/1940-6207.CAPR-12-0449-T. Epub 2013 Jan 30. Cancer Prev Res (Phila). 2013. PMID: 23365133 Free PMC article.
-
Succinobucol, a Lipid-Lowering Drug, Protects Against 3-Nitropropionic Acid-Induced Mitochondrial Dysfunction and Oxidative Stress in SH-SY5Y Cells via Upregulation of Glutathione Levels and Glutamate Cysteine Ligase Activity.Mol Neurobiol. 2016 Mar;53(2):1280-1295. doi: 10.1007/s12035-014-9086-x. Epub 2015 Jan 27. Mol Neurobiol. 2016. PMID: 25619973
-
Notch signaling and neuronal death in stroke.Prog Neurobiol. 2018 Jun-Aug;165-167:103-116. doi: 10.1016/j.pneurobio.2018.03.002. Epub 2018 Mar 21. Prog Neurobiol. 2018. PMID: 29574014 Free PMC article. Review.
References
-
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, Sawa A. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. - PubMed
-
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. - PubMed
-
- Cho Y, Griswold A, Campbell C, Min KT. Individual histone deacetylases in Drosophila modulate transcription of distinct genes. Genomics. 2005;86:606–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
