CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function
- PMID: 19251297
- PMCID: PMC2667870
- DOI: 10.1016/j.virol.2009.01.044
CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function
Abstract
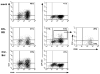
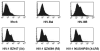
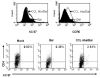
NK cells mediate the innate immune response, and HIV-infected individuals demonstrate altered NK cell phenotype and function. We find that CD4+ NK cells are susceptible to HIV infection; this could account for the NK cell dysfunction seen in HIV-infected individuals. CD4+ NK cells express CXCR4 and can be infected with X4-tropic viruses and some primary R5-utilizing viral isolates. Treatment with the CXCR4 ligands AMD3100 and SDF-1alpha partially blocks infection with X4-tropic virus, treatment with anti-CCL Igs upregulates CCR5 surface expression and enables infection with HIV-Bal. HIV infection of NK cells results in CD4 downregulation and the production of infectious virus. HIV-infected CD4+ NK cells mediate NK cell cytotoxicity, however, HIV infection is associated with decreased chemotaxis towards IL-16. Thus, HIV infection of CD4+ NK cells could account for the NK cell dysfunction observed in HIV-infected individuals. Furthermore infected NK cells could serve as a viral reservoir of HIV in vivo.
Figures

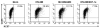
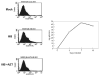
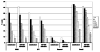

Similar articles
-
Differential effects of R5 and X4 human immunodeficiency virus type 1 infection on CD4+ cell proliferation and activation.J Gen Virol. 2005 Apr;86(Pt 4):1171-1179. doi: 10.1099/vir.0.80674-0. J Gen Virol. 2005. PMID: 15784911
-
Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy.Proc Natl Acad Sci U S A. 2002 May 14;99(10):7015-20. doi: 10.1073/pnas.102672999. Proc Natl Acad Sci U S A. 2002. PMID: 12011460 Free PMC article.
-
Differences in molecular evolution between switch (R5 to R5X4/X4-tropic) and non-switch (R5-tropic only) HIV-1 populations during infection.Infect Genet Evol. 2010 Apr;10(3):356-64. doi: 10.1016/j.meegid.2009.05.003. Epub 2009 May 14. Infect Genet Evol. 2010. PMID: 19446658
-
Relationships Between HIV-Mediated Chemokine Coreceptor Signaling, Cofilin Hyperactivation, Viral Tropism Switch and HIV-Mediated CD4 Depletion.Curr HIV Res. 2019;17(6):388-396. doi: 10.2174/1570162X17666191106112018. Curr HIV Res. 2019. PMID: 31702526 Review.
-
HIV-1 coreceptor usage, transmission, and disease progression.Curr HIV Res. 2003 Apr;1(2):217-27. doi: 10.2174/1570162033485357. Curr HIV Res. 2003. PMID: 15043204 Review.
Cited by
-
DCs and NK cells: critical effectors in the immune response to HIV-1.Nat Rev Immunol. 2011 Mar;11(3):176-86. doi: 10.1038/nri2935. Nat Rev Immunol. 2011. PMID: 21350578 Free PMC article. Review.
-
HIV-1 Reservoirs During Suppressive Therapy.Trends Microbiol. 2016 May;24(5):345-355. doi: 10.1016/j.tim.2016.01.006. Epub 2016 Feb 12. Trends Microbiol. 2016. PMID: 26875617 Free PMC article. Review.
-
IL-17 mediates neutrophil infiltration and renal fibrosis following recovery from ischemia reperfusion: compensatory role of natural killer cells in athymic rats.Am J Physiol Renal Physiol. 2017 Mar 1;312(3):F385-F397. doi: 10.1152/ajprenal.00462.2016. Epub 2016 Nov 16. Am J Physiol Renal Physiol. 2017. PMID: 27852609 Free PMC article.
-
Respiratory Syncytial Virus Infects Primary Neonatal and Adult Natural Killer Cells and Affects Their Antiviral Effector Function.J Infect Dis. 2019 Feb 15;219(5):723-733. doi: 10.1093/infdis/jiy566. J Infect Dis. 2019. PMID: 30252097 Free PMC article.
-
Viral Infection of Human Natural Killer Cells.Viruses. 2019 Mar 12;11(3):243. doi: 10.3390/v11030243. Viruses. 2019. PMID: 30870969 Free PMC article. Review.
References
-
- Ahmad A, Menezes J. Positive correlation between the natural killer and gp 120/41-specific antibody-dependent cellular cytotoxic effector functions in HIV- infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(2):115–9. - PubMed
-
- Ahmad R, Sindhu ST, Tran P, Toma E, Morisset R, Menezes J, Ahmad A. Modulation of expression of the MHC class I-binding natural killer cell receptors, and NK activity in relation to viral load in HIV-infected/AIDS patients. J Med Virol. 2001;65(3):431–40. - PubMed
-
- Bernstein HB, Jackson RW, Anderson J, Kinter AL. The effect of elective cesarean delivery and intrapartum infection on fetal lymphocyte activation and susceptibility to HIV infection. Am J Obstet Gynecol. 2002;187(5):1283–9. - PubMed
-
- Bernstein HB, Plasterer MC, Schiff SE, Kitchen CM, Kitchen S, Zack JA. CD4 expression on activated NK cells: ligation of CD4 induces cytokine expression and cell migration. J Immunol. 2006;177(6):3669–76. - PubMed
-
- Biswas P, Mantelli B, Sica A, Malnati M, Panzeri C, Saccani A, Hasson H, Vecchi A, Saniabadi A, Lusso P, Lazzarin A, Beretta A. Expression of CD4 on human peripheral blood neutrophils. Blood. 2003;101(11):4452–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

