Cloning, characterization, and expression analysis of the novel acetyltransferase retrogene Ard1b in the mouse
- PMID: 19246321
- PMCID: PMC2849813
- DOI: 10.1095/biolreprod.108.073221
Cloning, characterization, and expression analysis of the novel acetyltransferase retrogene Ard1b in the mouse
Abstract
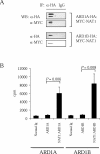
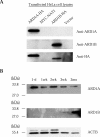
N-alpha-terminal acetylation is a modification process that occurs cotranslationally on most eukaryotic proteins. The major enzyme responsible for this process, N-alpha-terminal acetyltransferase, is composed of the catalytic subunit ARD1A and the auxiliary subunit NAT1. We cloned, characterized, and studied the expression pattern of Ard1b (also known as Ard2), a novel homolog of the mouse Ard1a. Comparison of the genomic structures suggests that the autosomal Ard1b is a retroposed copy of the X-linked Ard1a. Expression analyses demonstrated a testis predominance of Ard1b. A reciprocal expression pattern between Ard1a and Ard1b is also observed during spermatogenesis, suggesting that Ard1b is expressed to compensate for the loss of Ard1a starting from meiosis. Both ARD1A and ARD1B can interact with NAT1 to constitute a functional N-alpha-terminal acetyltransferase in vitro. The expression of ARD1B protein can be detected in mouse testes but is delayed until the first appearance of round spermatids. In a cell culture model, the inclusion of the long 3' untranslated region of Ard1b leads to reduction of luciferase reporter activity, which implicates its role in translational repression of Ard1b during spermatogenesis. Our results suggest that ARD1B may have an important role in the later course of the spermatogenic process.
Figures



Similar articles
-
Organization and chromosomal localization of the murine Testisin gene encoding a serine protease temporally expressed during spermatogenesis.Eur J Biochem. 2001 Mar;268(5):1250-8. doi: 10.1046/j.1432-1327.2001.01986.x. Eur J Biochem. 2001. PMID: 11231276
-
The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation.Mol Cell Biol. 2010 Apr;30(8):1898-909. doi: 10.1128/MCB.01199-09. Epub 2010 Feb 12. Mol Cell Biol. 2010. PMID: 20154145 Free PMC article.
-
N-acetyltransferase ARD1-NAT1 regulates neuronal dendritic development.Genes Cells. 2008 Nov;13(11):1171-83. doi: 10.1111/j.1365-2443.2008.01235.x. Genes Cells. 2008. PMID: 19090811
-
An evolutionarily conserved N-terminal acetyltransferase complex associated with neuronal development.J Biol Chem. 2003 Oct 10;278(41):40113-20. doi: 10.1074/jbc.M301218200. Epub 2003 Jul 29. J Biol Chem. 2003. PMID: 12888564
-
Identification and characterization of functional rat arylamine N-acetyltransferase 3: comparisons with rat arylamine N-acetyltransferases 1 and 2.J Pharmacol Exp Ther. 2006 Oct;319(1):369-75. doi: 10.1124/jpet.106.108399. Epub 2006 Jul 7. J Pharmacol Exp Ther. 2006. PMID: 16829624
Cited by
-
Regulation of HBEGF by Micro-RNA for Survival of Developing Human Trophoblast Cells.PLoS One. 2016 Oct 4;11(10):e0163913. doi: 10.1371/journal.pone.0163913. eCollection 2016. PLoS One. 2016. PMID: 27701455 Free PMC article.
-
Expression of human NAA11 (ARD1B) gene is tissue-specific and is regulated by DNA methylation.Epigenetics. 2011 Nov;6(11):1391-9. doi: 10.4161/epi.6.11.18125. Epub 2011 Nov 1. Epigenetics. 2011. PMID: 22048246 Free PMC article.
-
De novo missense mutations in the NAA10 gene cause severe non-syndromic developmental delay in males and females.Eur J Hum Genet. 2015 May;23(5):602-9. doi: 10.1038/ejhg.2014.150. Epub 2014 Aug 6. Eur J Hum Genet. 2015. PMID: 25099252 Free PMC article.
-
Metabolic regulation of proteome stability via N-terminal acetylation controls male germline stem cell differentiation and reproduction.Nat Commun. 2023 Oct 23;14(1):6737. doi: 10.1038/s41467-023-42496-9. Nat Commun. 2023. PMID: 37872135 Free PMC article.
-
Long-term vitamin A deficiency induces alteration of adult mouse spermatogenesis and spermatogonial differentiation: direct effect on spermatogonial gene expression and indirect effects via somatic cells.J Nutr Biochem. 2013 Jun;24(6):1123-35. doi: 10.1016/j.jnutbio.2012.08.013. Epub 2012 Dec 17. J Nutr Biochem. 2013. PMID: 23253600 Free PMC article.
References
-
- Driessen HP, de Jong WW, Tesser GI, Bloemendal H.The mechanism of N-terminal acetylation of proteins. CRC Crit Rev Biochem 1985; 18: 281–325. - PubMed
-
- Polevoda B, Sherman F.Nα-terminal acetylation of eukaryotic proteins. J Biol Chem 2000; 275: 36479–36482. - PubMed
-
- Polevoda B, Sherman F.Composition and function of the eukaryotic N-terminal acetyltransferase subunits. Biochem Biophys Res Commun 2003; 308: 1–11. - PubMed
-
- Whiteway M, Szostak JW.The ARD1 gene of yeast functions in the switch between the mitotic cell cycle and alternative developmental pathways. Cell 1985; 43: 483–492. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

